Researchers demonstrate new approaches to IR microscopy
A variety of novel microspectroscopic techniques are driven directly by advancements in laser technologies, such as how compact mode-locked fiber lasers have reduced barriers to multiphoton fluorescence microscopy1 and how tunable quantum cascade lasers (QCLs) are revolutionizing infrared (IR) microscopy by eliminating the need for complex interferometric detection.2
While both of these microscopy techniques are extremely powerful, they also suffer from their own unique drawbacks. For example, fluorescence microscopy can provide extremely high-resolution chemical images, but lacks the chemical specificity of vibrational techniques. On the other hand, IR microscopy offers exceptional chemical specificity, but the long wavelengths associated with the IR region limit the spatial resolution to a few microns.
At this year’s SciX conference in Providence, RI, Minghe Li, from the Simpson Laboratory for Nonlinear Optics at Purdue University (West Lafayette, IN), presented a fascinating talk on how these two approaches can be combined to produce sub-diffraction-limited IR images.3 Interestingly, it turned out that Garth Simpson’s group at Purdue was not the only one working on this problem. Ji-Xin Cheng’s team at Boston University (Boston, MA) was also independently developing a virtually identical methodology at the same time. When each group realized what the other was doing, they chose to coordinate their manuscripts’ release instead of trying to scope each other. This led to the publication of two articles with the same title—“Fluorescence-Detected Mid-Infrared Photothermal Microscopy” (F-PTIR)—published in parallel in the Journal of the American Chemical Society (JACS) in July 2021.4,5
To understand the significance of their new approach to IR microscopy, it is helpful to first explore photothermal IR microscopy in general. Since IR absorption bands directly correlate to vibrational energy states of a molecule, and temperature is loosely defined as the average kinetic energy of a collection of molecules, it should come as no surprise that when a sample absorbs IR light, it heats up. Therefore, one can define photothermal IR microscopy as any technique that detects localized temperature changes in a sample as a function of wavelength instead of direct IR absorption or reflection.
IR-induced photothermal changes in samples have been used for more than 20 years to overcome the diffraction limit of IR microscopy, most notably using atomic force microscopy (AFM).6,7 AFM uses a fine-tipped cantilever scanned over a sample, mapping the repulsive force between the electron clouds of the sample and the AFM tip. When performing AFM-IR, a tunable IR laser (typically a QCL) is focused on the same spot as the AFM tip. As the laser wavelength is scanned across the sample, whenever it corresponds to an IR absorption band, it causes the sample to heat up locally and expand, which is detected by vibrations of the cantilever. AFM-IR provides exceptional spatial resolution, but the system complexity and prolonged measurement time make it less desirable for widespread implementation.
Another common approach to photothermal IR microscopy is to use a visible wavelength laser to measure the changes in the index of refraction because of photothermal IR heating. This methodology, known as optically detected photothermal IR (O-PTIR) microscopy, allows for submicron resolution without the cost and complexities of AFM techniques. But since thermally induced refractive index changes are minimal (~0.01%/°C for water8), it is challenging to detect accurately. Furthermore, since it’s not possible to directly measure the thermal lensing at the laser’s focus, O-PTIR microscopes measure the darkfield response. While effective, it makes the signal more difficult to interpret since the darkfield is also highly dependent on the natural scattering from the structure of the sample itself. Still, until the recent work done at Purdue and Boston, this was the only viable method for high-resolution optical IR microscopy. It is important to note that despite these challenges, O-PTIR microscopy has seen a relatively high degree of commercial success, particularly after the founding of Photothermal Spectroscopy Corp. (Santa Barbara, CA) in 2018.
The advantages of F-PTIR
Even though the temperature dependence of fluorescence has been known since the 1950s,9 it wasn’t until the recent work done at Purdue and Boston that anyone has attempted to use thermal fluorescence quenching for photothermal IR microscopy. According to the recent JACS article,3 “Compared to refractive index changes, the change in fluorescence quantum efficiency can be quite large, routinely changing by ∼2−3%/°C for tryptophan and ∼1−2%/°C for aqueous solutions of rhodamine-B, corresponding to an ∼100-fold higher relative change than refractive index detection.”5
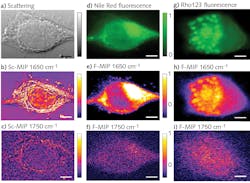
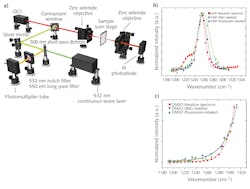
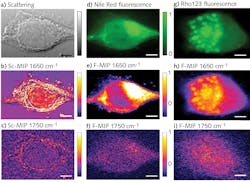
To demonstrate the efficacy of F-PTIR, the team at Purdue used both traditional fluorescence tagging and multiphoton fluorescence. Both setups utilized a novel QCL array from Pendar Technologies (Cambridge, MA), which consisted of 32 independently addressable emitters ranging from 1190 to 1330 cm-1 (7.52–8.62 µm). The fluorophore-based measurements used a copropagating continuous-wave (CW) 532 nm laser from SLOC Lasers (see Fig. 1a), and the multiphoton measurements used a counterpropagating 532 nm frequency-doubled, mode-locked fiber laser from Fianium (Southampton, UK).
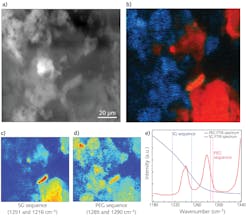
As a proof-of-principle experiment, the researchers measured thin films of dimethylformamide (DFM) and dimethyl sulfoxide (DMSO), each laced with rhodamine-6G (R6G) and fluorescein fluorophores. The recorded F-PTIR spectra (see Fig. 1b and 1c) showed excellent agreement with the IR spectra in the NIST standard reference database for both samples and fluorophores. After demonstrating the feasibility of F-PTIR, the Purdue team went on to successfully image silica gel (SG) particles labeled with R6G. Using edge analysis, they determined the spatial resolution to be 1.7 µm—well below the diffraction limit. They followed up by successfully producing a high-resolution F-PTIR image of a mixture of polyethylene glycol (PEG), SG, and R6G (see Fig. 2). Following their success with traditional fluorescence, the Simpson group switched its efforts to the multiphoton approach, producing label-free F-PTIR images of tryptophan microcrystals as well as dispersions of ritonavir in polyvinylpyrrolidone/vinyl acetate (PVPVA).It should be noted that Yi Zhang et al. use slightly different terminology in the article, referring to O-PTIR as Sc-MIP and F-PTIR as F-MIP.
Looking to the future
While F-PTIR is extremely promising, it is not likely to replace either O-PTIR or AFM-IR—instead, it is complementary.
“I think that there is a lot of opportunities to couple O-PTIR and F-PTIR,” Simpson says. “I think they are very highly complementary.” He also highlighted the advantages of using ultrafast sources for label-free fluorescence and discussed the potential to detect second-harmonic generation (SHG) simultaneously. He points out, too, that SHG can determine if there is a crystalline domain, while F-PTIR tells the structure and composition of that domain.
While it is still early in the development of this new technique, it is evident that F-PTIR has the potential to revolutionize the high-resolution IR microscopy market.
REFERENCES
1. R. V. Chimenti, Laser Focus World, 56, 12, 29–31 (Dec. 2020); https://bit.ly/3BZfDO8.
2. R. V. Chimenti, Laser Focus World, 57, 7, 43–46 (Jul. 2021); https://bit.ly/3EXuf2v.
3. M. Li et al., arXiv (2021); https://arxiv.org/abs/2104.02900.
4. Y. Zhang et al., JACS, 143, 11490–11499 (2021); doi:10.1021/jacs.1c03642.
5. Y. Zhang et al., JACS, 143, 29, 10809–10815 (2021); doi:10.1021/jacs.1c03642.
6. A. Centrone, Annu. Rev. Anal. Chem., 8, 1, 101–126 (2015).
7. A. Dazzi and C. B. Prater, Chem. Rev., 117, 7, 5146–5173 (2017).
8. G. Abbate, U. Bernini, E. Ragozzino, and F. Somma, J. Phys. D: Appl. Phys., 11, 8, 1167 (1978).
9. E. J. Bowen and J. Sahu, J. Phys. Chem., 63, 1, 4–7 (1959).
About the Author
Robert V. Chimenti
Director, RVC Photonics LLC
Robert V. Chimenti is the Director of RVC Photonics LLC (Pitman, NJ), as well as a Visiting Assistant Professor in the Department of Physics and Astronomy at Rowan University (Glassboro, NJ). He has earned undergraduate degrees in physics, photonics, and business administration, as well as an M.S. in Electro-Optics from the University of Dayton. Over a nearly 20-year career in optics and photonics, he has primarily focused on the development of new laser and spectroscopy applications, with a heavy emphasis on vibrational spectroscopy. He is also very heavily involved in the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), where he has served for several years as the Workshops Chair for the annual SciX conference and will be taking over as General Chair for the 2021 SciX conference.