Hyperspectral imaging device helps debunk wound healing
Hyperspectral imaging (HSI) devices, calibrated to new National Institute of Standards and Technology (NIST; Gaithersburg, MD) standard reference spectra, bring forth new insight on the physiology of tissue injury and healing. Currently, many physicians would prefer to use completely noninvasive HSI methods to measure the amount of oxygen (a key factor in wound healing) in widely affected tissue, which cannot be assessed without biopsies or transcutaneous techniques limited to a single point.
"Hyperspectral imaging isn't being used routinely in the clinic yet because standards aren't in place," says David Allen of the Sensor Science Division at NIST's Physical Measurement Laboratory (PML). Individual researchers doing their own experiments show positive results. But when you start comparing an instrument in one lab to an instrument in another, the data are typically inconsistent, he says. Factors limiting repeatable results include biological variability (variations in skin pigmentation, tissue density, lipid content, and blood volume changes), and sensor variability related to calibration and best practices in the measurement protocol. While the biological variability is beyond researchers' control, the sensor variability can be minimized.
In pursuit of that goal, Allen and colleagues have now produced the first prototype "digital tissue phantoms" derived from bench-top simulations and in-vivo wound imaging. The new PML digital tissue phantoms (DTPs) are a set of specific spectral signatures and images that correspond to different states of hemoglobin oxygenation due to ischemia (inadequate blood flow), which ultimately result in cell death due to oxygen deprivation.
Ultimately, more extensive and clinically validated versions of the phantoms can be used to calibrate spectral imaging devices of various kinds. Those devices will be able to detect the telltale spectral evidence of ischemia, revascularization, assorted pathologies, and other conditions suggestive of tissue viability at a wound site, on a microvascular scaleâeven during surgery.
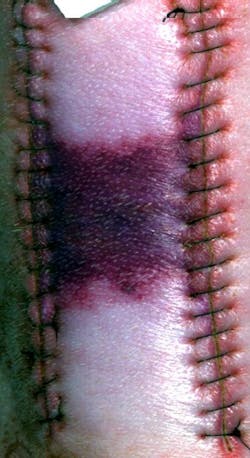
But in order for that to happen, there must be a well-characterized and clinically validated correspondence between particular spectral signature and particular tissue conditions. To tackle this, Allen and scientists from Ron Xuâs group at the Ohio State University (OSU) Biomedical Engineering Department (Columbus, OH) imaged carefully manipulated ischemic wounds in an anesthetized pigâthe first time anybody has looked at a porcine skin flap animal model hyperspectrally, says Allen.
On two areas of the animal's back, flaps of skin were raised, silicone-plastic sheets were placed beneath the skin to inhibit reperfusion, and the incisions were closed. On adjacent areas were a skin flap without plastic sheets and an untreated control area. Allen scanned all the areas with a highly sensitive hyperspectral imager, using illumination from a standard broadband light source. In this work, a typical scan encompassed 240 different wavelengths, spaced about 2 nm apart, ranging from 400 to 880 nm. As a reflectance reference, each image also included light from a NIST-traceable standard diffuser. Readings at each wavelength were then stacked into "data cubes" for each scanned position. The group found that they could reproduce the tissue spectrumâincluding the oxygenation level of hemoglobinâto within one standard deviation, well within the natural variability of the tissue, says Allen.
Then, Allen's group received NIST funding to develop standards for the field. One part of their award supported use of a PML device called the hyperspectral image projector (HIP), developed by Joe Rice and others at NIST, which reproduces complex spectral-spatial images very accurately by precision control of digital micromirror devices.
When medically significant hyperspectral scenes are projected, they are referred to as digital tissue phantoms. They allow realistic medical scenes to be produced repeatedly without the variability and expense that would occur if the medical procedure was repeated every time that a hyperspectral image was evaluated. Already Allen's group has been able to generate HIP-projectable spectral signatures that correspond to different levels of oxygenation in tissue.
Currently, accumulating the data for DTPs is a time-consuming process: Each scan takes from tens of seconds to a minute or more. But the group is working on 'snapshot' hyperspectral scans for real-time imaging, including video, says Allen. "Eventually, we want to get to the point at which you can see the blood perfusing through the tissue," he explains.
In addition to optical medical imaging, Allen is also investigating HSI's potential in environmental and defense applications, such as diseases of coral reefs and the detection of hidden explosive devices.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Follow OptoIQ on your iPhone; download the free app here.
Subscribe now to BioOptics World magazine; it's free!