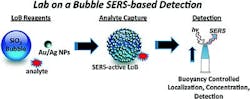
SERS-driven 'lab on a bubble' shows potential for point-of-care diagnostics
| Hollow silicon dioxide spheres (top) covered with gold nanoparticles grab an analyte from solution (center) and then float to the top (bottom) for easy characterization. (Image courtesy of the Journal of the American Chemical Society) |
Researchers from the University of Wyoming (Laramie, WY), coatings and materials company iFyber (Ithaca, NY), and colleagues have developed a quicker method to concentrate samples that relies on beads that float in a bubble, which could detect biological molecules using immunoassays for potential use in point-of-care diagnostics, as reported by Chemical & Engineering News.
The team used surface-enhanced Raman scattering (SERS) spectroscopy to detect chemicals using paramagnetic beads that attach to gold nanoparticles only in the presence of a compound of interest. When the researchers captured the beads with a magnet and shone a laser on them, the compound adsorbed onto the gold nanoparticles to produce a characteristic SERS signal.
But a magnetic fieldâs strength falls dramatically with distance, says Keith T. Carron of the University of Wyoming. To speed things up the team developed a "lab on a bubble," which involves hollow 50-μm-diameter silica spheres covered with 50-nm-diameter gold nanoparticles to capture certain chemicals in solution. When the researchers mix the nanoparticle-covered spheres into a sample, they rise to the surface; therefore, sample volume is not an issue. Then, the researchers can probe them with a Raman laser.
The team used their "lab-on-a-bubble" method to measure known concentrations of cyanide ions, which enabled detection of the ions down to 173 ppt--a similar sensitivity to using gold nanoparticles alone. But the SERS signal from the bubble method has considerably less noise, Carron says, because the spheres stay in a relatively small volume. Without their flotation devices, he explains, nanoparticles wouldnât concentrate in the laserâs beam and would drift in and out of the detection area, which leads to signal noise. The bubbles also produce about 28 times larger SERS signals than gold nanoparticles do alone because the spheres prevent the gold from aggregating.
The team's work has been published in the Journal of the American Chemical Society. For more information, please visit http://pubs.acs.org/doi/abs/10.1021/ja208463f.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Follow OptoIQ on your iPhone; download the free app here.
Subscribe now to BioOptics World magazine; it's free!