Imaging technologies take charge in the war on cancer
Quantitative spectroscopy, elastic light scattering, optical frequency-domain imaging, in vivo fluorescent imaging, vibrational microspectroscopy—these and similar optical techniques have made molecular imaging the fastest-growing approach to understanding and diagnosing cancers today.
Researchers in biomedicine and physical science highlighted their efforts last November at Optical Imaging for Medicine and Biology: Applications in Cancer Detection, a one-day seminar hosted by Boston University’s College of Engineering (Boston, MA). Channeling a World War II speech by Winston Churchill in her summary of the state of progress in the development of optical technologies for cancer detection, Karen Antman, dean and provost of the Boston University School of Medicine, noted, “We are not at the end, or even the beginning of the end. But we are at the end of the beginning.”
Spectroscopy underpins many of the new approaches. As Michael Feld, professor of physics at Massachusetts Institute of Technology (MIT; Cambridge, MA), put it, researchers are now studying “the color of cancer.” Feld’s team at MIT has devised a quantitative spectroscopy technique for detecting cervical dysplasia—an otherwise invisible early indicator of cervical cancer—at the surface level.
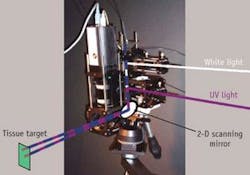
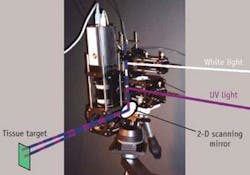
“We focus on fluorophores and other items present in the tissue and we study only one millimeter from the surface, in which 90% of all cancers develop,” he explains. The team has developed what Feld calls a “virtual probe” that delivers first white light and then ultraviolet light to the target tissue, for reflection followed by fluorescence (see figure). “We image a light spot on the target surface with a raster scan, so that we can create an entire large image composed of 1 mm pixels,” he says.
Studies to date have shown that the spectroscopic map that the technology produces can outline areas of high-grade cervical dysplasia. The group plans to expand its approach to cancers of the oral cavity and to introduce it in endoscopes to study progenitors of throat, lung, colon, and esophageal cancers.
Elastic-scattering spectroscopy
Across the Charles River, Irving Bigio, professor of biomedical engineering and electrical and computer engineering at Boston University, is developing elastic-scattering spectroscopy (ESS) to sense subcellular changes caused by impending cancers in the colon and other organs. He began working with ESS to develop a smart, noninvasive form of colonoscopy that is user-friendly and clinically practical.
“ESS is sensitive to scattering changes in subcellular morphology,” he says. “The method senses those morphology changes in a semiquantitative manner, without actually imaging the microscopic structure.”
Bigio’s group has incorporated a light source and a spectrometer into a flexible fiber-optic probe that can be guided through the colon, esophagus, or other organ to detect changes in tissue that might indicate dysplasias or cancers. The fiber is also integrated into conventional endoscopy equipment, replete with forceps and a snare. That permits doctors to snip out tissues for biopsy as they spot suspicious-looking areas rather than, as they do now, sampling on a random basis. So far, Bigio reports, this real-time optical biopsy technique has shown promise in detecting dysplasias in the esophagus and polyps in the colon.
“We’re getting to the point where this is ready for prime time,” he says.
Other optical-imaging techniques are also playing a role in the struggle to spot cancers earlier than ever before. Gary Tearney of Massachusetts General Hospital’s Wellman Center for Photomedicine (Boston) reports that newly developed optical-frequency-domain imaging (OFDI—an ultrafast form of optical coherence tomography), which has detected early signs of coronary artery disease in live pigs, might also improve the diagnosis and management of cancer in human patients.
Optical-frequency-domain imaging provides a rapid means of imaging the interiors of blood vessels and other organs. A laser tip emits an infrared beam that changes frequencies as it rotates inside a fiber-optic catheter traveling through the appropriate organs. The probe captures reflections from the organ’s interior. Fourier transforming converts the reflections into images that can reveal incipient diseases such as indications of dysplasias in the esophagus. After successful studies on the coronary arteries of live pigs, Tearney’s team has started the first human trials of the technology.
Other groups at the seminar reported research at the preclinical level. VisEn Medical, a spin-off company from Massachusetts General Hospital, is combining near-infrared probes with fluorescence molecular tomography to study the progress of cancers and other diseases in mice, according to Jeff Peterson, director of applied biology at VisEn. In addition, a team at Northeastern University headed by chemistry and chemical biology professor Max Diem is using infrared and Raman microscopy to create a machine-based technique for diagnosing cancers in biopsied tissue. —PG