As the old British saying goes, “there’s many a slip ’twixt cup and lip,” especially when the cup is a flask in a pharmaceutical laboratory and the lip belongs to a patient ingesting a newly produced medication. Taking an approved drug through process development, scaling it up to manufacturing, and ensuring the consistent quality of the manufacturing procedure presents a series of problems, each of which has the potential to disrupt the drug’s entire future, or at least reduce its market share.
Current testing methods are typically slow and cumbersome. But pharmaceutical companies now see the promise of faster, more accurate means of quality assurance. The new vehicle? Spectroscopy.
Several issues can hinder the smooth progress from the lab to the clinic of a drug designed for administration in pill or capsule form. The chemical make-up of the components can change during the process. Formulations can convert to polymorphs—versions with the same chemical formulae but different crystalline structures and biochemical and medicinal actions. Physical cracks can appear inside a pill’s core. Coatings designed to permit controlled release of medications can vary in density and thickness or allow moisture to pass through, thereby reducing the precision of release. Adhesion between the layers of bilayered pills, which consist of two formulations designed to act in combination, can be compromised. And several issues can affect the long-term stability of individual pills.
In each of these cases fast, accurate, nondestructive testing can alert manufacturers to potential problems. Three spectroscopic methods—terahertz spectroscopy, spatially offset Raman spectroscopy, and laser-induced breakdown spectroscopy—have shown particular promise in monitoring pharmaceutical scale-up and manufacture.
3-D imagery
Terahertz spectroscopy operates between the microwave and optical regions, from 30 µm to 3 mm wavelength. The radiation is sensitive to the vibrations of noncovalent chemical bonds—intermolecular vibrations or vibrations of large units in molecules—but not to those of discrete bonds. For the pharmaceutical industry, terahertz spectroscopy offers the advantage of providing chemical information in three dimensions about the molecular contents of pills in solid and capsule form that are normally invisible to conventional monitoring methods. The fact that water strongly absorbs the radiation is an added plus. It makes it easy to detect moisture in pills.
TeraView (Cambridge, England) has emerged as a leader in the application of terahertz spectroscopy to the pharmaceutical industry’s needs. The company has carried out case studies in collaboration with pharmaceutical companies and the U.S. Food and Drug Administration (FDA) that have helped to illustrate the technology’s benefits to big pharma in terms of reducing development time, ensuring rapid scale-up into manufacture, and maintaining in-spec product with reduced risk of regulatory noncompliance.
“The main benefits we offer to pharma manufacturing are improving process understanding in terms of risking product scale-up and transfer and then being able to provide ongoing monitoring for quality assurance,” says Maireadh Pedersen, TeraView’s vice president of business development in pharmaceuticals. “We work with customers to build a model where we measure specific attributes associated with the dosage form that are linked to the performance of the product; none of these can be measured nondestructively with other technologies. We then use terahertz to measure the tablets resulting from, for example, a process design of experiment (DOE). Terahertz allows more tablets to be measured much faster than using dissolution, so more accurate results can be obtained for the success of various points in the DOE.”
A key concern to pharmaceutical firms is dissolution—the tendency of coatings designed to release drugs at a steady rate inside the body to dissolve erratically. “Most of the case studies are driven by customer products that are failing dissolution in some way,” Pedersen says. “Dissolution tells you the drug release rate but does not identify the source of the change and variation seen between tablets. Terahertz imaging allows you to map the tablet and its structure and then correlate to its dissolution profile or some other performance indicator, such as tablet integrity or hardness.”
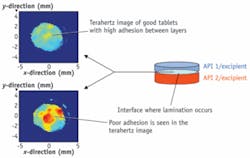
A study reported last year by scientists from the FDA, TeraView, and pharmaceutical firm Sandoz reported that terahertz pulsed imaging (TPI) accurately mapped the thicknesses of Asacol delayed-release tablets, which dissolve in the intestines to treat ulcerative colitis. A more recent study, by researchers at New Zealand’s University of Otago showed that measuring the density of coatings—another capability of TPI—provides a valuable indication of dissolution time. The same study showed that TPI yields information more significant to scale up sustained-release pills than current measurement methods. Pharmas are also using terahertz imaging to study the effects of accelerated stability on pills.“We can pick out changes occurring in the image early on in the process that can lead to an understanding of where the product is failing,” Pedersen explains. In one example, a client thought that a coating was failing and allowing moisture into the pills, which caused them to degrade. “We were able to monitor the product in real time and see that the failure was linked to degradation of an excipient in the tablet core, linked to temperature.”
GlaxoSmithKline and Pfizer have used TeraView’s technology to define the integrity of pills’ interiors during scale-up. They have also applied it to study the effect of changing spray rates on film coating, and to measure formulation effects on the adhesion between the layers of bilayer pills. Other TeraView clients include AstraZeneca and Takeda. “And obviously,” Pedersen says, “we have worked with the FDA on a couple of products.”
High chemical specificity
Researchers at another British facility, the Science and Technology Facilities Council’s Rutherford Appleton Laboratory, have developed another spectroscopic method with potential applications in pharmaceutical manufacturing. Spatially offset Raman spectroscopy (SORS) takes advantage of Raman spectroscopy’s high chemical specificity, which exceeds that of near infrared absorption (NIR) spectroscopy and compares with that of terahertz technology. “The technique is expected to replace the existing, less chemically specific NIR absorption spectroscopy in applications where higher chemical specificity or noninvasive probing—through capsules, for example—is required,” says Pavel Matousek, project leader.
Conventional Raman spectroscopy has the disadvantage of blanketing signals from pills’ interiors with those from their surfaces. SORS collects Raman signals offset from the point of illumination on the sample’s surface. The laterally offset spectra contain different relative contributions from layers at different depths. That serves to suppress interfering signals from the surface layers. “The technique has a potential to improve sensitivity and accuracy in critical process analytical technology applications in general,” Matousek says. “As such, it promises to reduce waste stemming from failed products and to improve the efficiency of the manufacturing process.”
Several manufacturers have expressed interest in the new technology. “Presently we collaborate with Pfizer, AstraZeneca, and GlaxoSmithKline, aiming at further optimizing the technique for the production line environment,” Matousek says. “Simultaneously, we are launching a new spinout, LiteThru Ltd., to deliver a commercial solution satisfying stringent requirements of production lines.”
From mining to pharma
Laser-induced breakdown spectroscopy (LIBS) has been a well-regarded technique for decades in mining, metallurgy, and environmental applications. Pharma Laser (Quebec, Canada) is collaborating with the National Research Council of Canada to develop the method as a means of identifying the molecular components of pills’ surfaces and interiors. A Nd:YAG laser first vaporizes the constituents of a sample and then atomizes and ionizes them. As the particles relax, they emit light at characteristic wavelengths, thereby facilitating their identification. The method requires no solvents, needs minimal sample preparation, and produces results within a minute.The technology has one obvious disadvantage: it destroys its samples. However, Pharma Laser sees it as part of the pharmacological armamentarium for testing the blend uniformity, blend distribution, and concentration, as well as the amount of active pharmaceuticals and functional excipients in pills. Taken together, terahertz spectroscopy, SORS, and LIBS form a triple play that offers pharmaceutical manufacturers significant improvements in scale-up and quality assurance.
About the Author
Peter Gwynne
Freelance writer
Peter Gwynne is a freelance writer based in Massachusetts; e-mail: [email protected].