U.S. FDA clears Optovue OCT angiography measurement technology
Optovue (Fremont, CA) has received U.S. FDA clearance of its AngioAnalytics optical coherence tomography angiography (OCTA) blood vessel measurement technology to help clinicians manage diseases that cause progressive blindness. The company also received clearance for its three-dimensional projection artifact removal (3D PAR) software, which improves OCTA image quality and enables accurate measurement and interpretation of OCTA images. AngioAnalytics and 3D PAR are commercially available in the U.S.
Related: Optovue develops higher-density OCT angiography system for ophthalmology
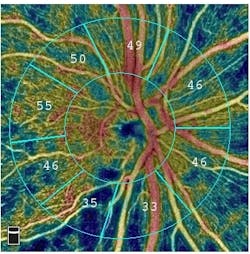
AngioAnalytics brings objective data and analysis to the company's commercially available AngioVue OCTA technology that provides high-resolution imaging of retinal blood vessels. Combined, the two technologies create color-encoded maps of the vessel densities of the retina or optic nerve, and provide analyses of areas where there is blood vessel loss (non-perfusion), abnormal blood vessel growth (flow area), and several parameters to assess change to the foveal avascular zone, an area of the retina profoundly affected by diabetic retinopathy. The AngioAnalytics software also provides trend analysis so that physicians can objectively monitor retinal and vascular changes caused by disease progression or from treatment.
Projection artifacts, inherent in OCTA technology from all manufacturers, occur when ghost images of blood vessels that exist in one retinal layer are projected onto other layers. The shadows from these vessels make it difficult to accurately interpret whether, and how much, abnormal vessel growth is actually present in the specific layers. 3D PAR technology is important for improving OCTA image quality, as it forms the basis for accurate qualitative image interpretation and reliable measurements that enable physicians to quickly document disease states and incorporate OCTA technology into their diagnostic armamentarium.
For more information, please visit www.optovue.com.