OPTICAL COHERENCE TOMOGRAPHY/CARDIOLOGY: Totally tubular: Cardiovascular OCT goes prime time
Recent FDA approval of LightLab's intravascular imaging system launched OCT's second clinical market. The technology represents an important advance for interventional cardiologists, and upcoming developments will keep the new market dynamic.
In early May, LightLab Imaging (Westford, MA) announced a breakthrough: receipt of the U.S. Food and Drug Administration's first clearance for a clinical optical coherence tomography (OCT) system to be applied to cardiovascular imaging.
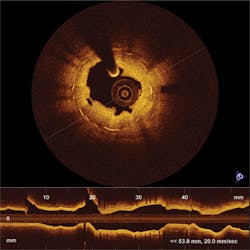
Figure 1. LightLab's FDA-approved C7 Dragonfly Imaging Catheter enables a surgeon to insert a high-resolution camera into the artery of a living person.
OCT has long been investigated for use to explore the interiors of coronary arteries. In fact, two other companies are now planning commercial release of OCT-based intravascular imaging systems: Terumo Corp. (Tokyo, Japan) and Volcano Corp. (San Diego, CA). And the technology has long been available in the U.S. for clinical ophthalmology; indeed, it has changed the practice. But LightLab's milestone marks the first time that American clinicians have access to OCT for an application other than eye care.
The FDA-approved products, the C7-XR Imaging System and companion C7 Dragonfly Imaging Catheter, allow a surgeon to insert a tiny, high-resolution camera into the artery of a living person during surgery —for instance, just prior to placement of a stent—to help understand structural detail and composition (see Fig. 1). By pulling the camera back through the artery, the clinician is able to see and measure important vessel characteristics otherwise invisible or difficult to observe with older intracoronary imaging modalities. The combined system had previously been approved in more than 35 countries in Europe and Asia, where it has been used by leading hospitals to perform high-resolution imaging of vessel and lumen morphology in thousands of coronary interventions.
How it helps
Like ultrasound, OCT technology images reflections from within tissue to generate cross-sectional views. But because the bandwidths of infrared light used for OCT are far higher than ultrasound, OCT's resolution is superior: 10 times that of intravascular ultrasound (IVUS).
"The market for imaging in cardiology is significant, and the market for OCT in this application has the potential to surpass that of ophthalmology," reports Strategies Unlimited (Mountain View, CA) in its study, Optical Coherence Tomography 2010: Technology, Applications, and Market.
The report cites four major cardiovascular applications for OCT systems:
- 1. Stent visualization
2. Vulnerable plaque assessment.
3. Drug and device development.
4. Peripheral artery disease visualization.
Figure 2. OCT highlights in-stent restenosis in a patient who has just undergone balloon dilatation. (Image courtesy LightLab Imaging.)
How it works
The C7-XR Imaging System and C7 Dragonfly Imaging Catheter incorporate LightLab's latest version of frequency domain (FD-OCT) technology—which is an evolution from the original OCT technology, time-domain (TD-OCT, see Fig. 4). Systems based on FD-OCT can acquire 100 frames/s, reaching pullback speeds up to 20 - 40 mm/s with the potential to scan 4- to 6-cm length epicardial coronary vessels in .5 s.1
Because the 1.3-µm light typically used in OCT systems cannot image through blood, producing a usable image necessitates flushing it from the area of interest. The accelerated pullback speed of the new system permits the use of a single, high-rate (~4 cc/s) bolus injection of contrast to produce a blood-free environment, thereby eliminating the need for balloon occlusion as was required by previous-generation TD-OCT systems, including LightLab's M2 TD-OCT.
LightLab OCT provides intravascular images with 15-µm axial resolution, about twice the size of a red blood cell. The C7-XR/catheter system sports a tunable laser light source with sweep range of 1,250 to 1,370 nm. The optical fiber is encapsulated within a rotating torque wire built in a rapid exchange 2.6-F catheter compatible with 6-F guides.1 It can generate more than 100,000,000 bytes of data in less than 3 seconds during a single 50-mm long coronary artery scan. Whereas TD-OCT systems moved a reference mirror to analyze tissue point by point, FD-OCT simultaneously analyzes thousands of data points quickly by scanning through the bandwidth of near-infrared wavelengths. Through its analysis, FD-OCT can report the composition of lesions—which may prompt changes in treatment plans and new approaches to coronary disease management.
Combining technologies
Two weeks after LightLab's FDA announcement, the company had more big news:
The global medical device company St. Jude Medical, Inc. (St. Paul, MN), had agreed to acquire the OCT developer for approximately $90 million in cash. According to the announcement, the acquisition "provides St. Jude Medical with a product platform to compete in and potentially expand the $500 million coronary imaging market." St. Jude Medical expects the OCT platform to contribute an additional $20 million in revenue to its cardiovascular business during the second half of 2010. St. Jude Medical boasts that it will be the first company to offer a portfolio that includes both OCT and Fractional Flow Reserve (FFR), a non-optical blood-flow measurement technology that has proven to enable better outcomes for stent placement.2 St. Jude says this combination will give physicians "comprehensive lesion assessment information."
Figure 3. Complete tissue coverage between the vessel lumen (dark area) and a half-dozen stent struts (brightest objects) in this OCT image demonstrate good healing. (Image courtesy LightLab Imaging.)
In response to the acquisition announcement, Scott Huennekens, President and CEO of LightLab competitor Volcano Corp., said the move "confirms the validity and potential utility of [OCT]." Volcano plans to use its own second-generation OCT catheter and system this year as part of VOILA, a U.S. regulatory trial. Meanwhile, the product received CE Mark approval in January, and is pending IDE approval with the FDA. The company expects commercial release in Europe in early 2011 and in the U.S. in mid-2011.
Volcano is known as a provider of IVUS products, and claims that 2,000 of its integrated IVUS systems are in cath labs today. Besides the integrated systems, which are actually built into cath labs the way factory-installed radios are built into cars, Volcano offers roll-around systems. The company plans to upgrade current customers with its OCT offering, and thereby be the only company offering both technologies. In the future, Hunnekens told BioOptics World, Volcano plans to combine OCT and IVUS on the same catheter, and in fact has a roadmap to accomplish this.
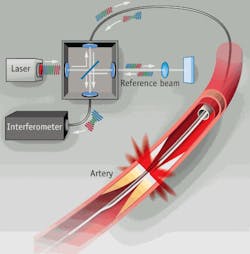
Figure 4. Time domain OCT (TD-OCT) creates an image by sending multiple, consecutive, near-infrared light beams into the tissue and measuring the intensity of the reflection by comparing it with a reference beam. This is repeated into deeper cells by adjusting the reference beam to correlate with a deeper point in the tissue. The data accumulated by these multiple beams broadcast to different depths at the same location is called a scan line. By contrast, frequency domain OCT (FD-OCT) collects all data along one scan line simultaneously by sending light of various wavelengths into the tissue to different depths. The reflected light pattern differs at each wavelength, and the reflected intensity of each wavelength corresponds to its depth in the tissue sample. This data is then analyzed by an interferometer to create the image. (Image courtesy LightLab Imaging.)
The combination of approaches will give interventional cardiologists more power: IVUS has a deeper penetration depth than OCT, and thus allows visualization of the entire thickness of the vessel wall. This can be important for stent sizing and visualization of very thick plaques.3
Volcano acquired its initial OCT technology through the purchase of CardioSpectra, Inc. in December 2007. Then, in 2009, Volcano acquired Axsun Technologies, supplier of tunable lasers for OCT imaging. The latter move added significant heat to the competitive situation since Axsun had signed an agreement less than a year earlier to supply its OCT engine to LightLab, which was formed in 1998 by the technology's co-inventors. (Litigation is still pending.) Now, Volcano says that the latest version of Axsun's light source is the proprietary technology that it hopes will give its product an edge in the long run. Volcano is withholding details until the commercial release of its system, but says the new technology overcomes fundamental limitations of laser-based designs for swept source OCT light sources. (Swept source is a subset of FD-OCT.) Based on a non-laser source of photons, it is called the High Definition Swept Source (HDSS) because it is expected to enable high resolution at unprecedented speeds in medical imaging systems (see Fig. 5). According to Axsun, HDSS has been tested at speeds of 200 kHz—approximately 4 to 10 times faster than earlier OCT imaging systems.
Figure 5. Axsun's new High Definition Swept Source (HDSS, at left) is the proprietary light source technology that Volcano says breaks fundamental speed limitations for producing intracoronary imagery (right). (Images courtesy Volcano Corp.)
Hunnekens reports that Volcano's system will be the fastest, with the smallest catheter, and enabling the fastest pullback. Though the company is not releasing size specifications on the catheter, Hunnekens says it will be able to track to more locations, including below the knee, and above the carotid artery and into the brain. And, he says, the small size will mean it won't get hung up on plaques. The pullback speed will cover some significant distance (Volcano won't yet say how much) in a single heartbeat—and thus generate imagery not subject to motion artifacting.
More for the future
Although Terumo Corp. did not respond to our requests for information, the company is apparently still making plans to manufacture and market a system based on optical frequency domain imaging (OFDI, a subset of FD-OCT), that was developed at Massachusetts General Hospital. That technology was featured in March 2009 on Good Morning America (http://bit.ly/9CylcQ).
Anyone seeing the more-recent morning show spot that highlighted LightLab's technology may have noticed the comment that OCT technology has not been shown to produce better results than IVUS (http://bit.ly/8YQggF). "We do not yet have a large-scale clinical study showing outcome benefit," says Craig Kelley, Director of Marketing for LightLab. "But based on the large collection of small studies that are currently published, we are very confident that we can produce proof of outcome enhancement."
REFERENCES
- Bezerra, HG et. al., J Am Coll Cardiol Intv, 2009; 2:1035-1046
- Pijls, NHP et. al., N Engl J Med 360:2024, May 7, 2009
- Optical Coherence Tomography 2010: Technology, Applications, and Market (Strategies Unlimited, January 2010)
More Brand Name Current Issue Articles
More Brand Name Archives Issue Articles