OPTICAL COHERENCE TOMOGRAPHY/BIOMEDICAL INSTRUMENTATION: Three-mode endoscopy for ovarian cancer detection
Yi Yang, Xiang Li, and Tianheng Wang
EDITOR'S NOTE: This article was adapted from Biomedical Optics Express, 2, 9, 2552–2561 (2011).
The reason ovarian cancer is the deadliest of all the gynecologic cancers has something to do with the fact that it begins to metastasize prior to the appearance of distinctive early symptoms—and much to do with a dearth of screening and diagnostic techniques that would enable early detection. During the past decade, prophylactic oophorectomy (PO)—that is, surgical removal of the ovaries as a preventive measure—has become the standard of care for high-risk women.1,2 But although PO reduces the risk of ovarian cancer by more than 50%, it also turns out to have significant detrimental effects, including increased mortality.3,4
Clearly, this application needs innovation. Ideally, an intraoperative device capable of measuring tissue optical absorption, generating scattering information, and imaging deep tissue structures could be applied intraoperatively.
A prototype intraoperative system that integrates optical coherence tomography (OCT), photoacoustic imaging (PAI, also called optoacoustic imaging), and pulse-echo ultrasound (US) has proven promising for characterizing ovarian tissue and detecting ovarian cancer. PAI provides an absorption map of blood vessel networks, OCT contributes high-resolution subsurface morphological imaging and provides optical scattering information, and US depicts deep tissue structures; the synergistic effect trumps the capabilities of each modality alone.
Three-part harmony
A high-resolution technique, OCT measures back-scattered light generated from an infrared light source directed to the tissues being studied—typically generating a resolution from several to tens of microns and a penetration depth of 1–3 mm.5 OCT technology has been developed during the past few years to image morphological features of pre-neoplastic or early neoplastic changes in early-stage ovarian cancer. 6-10 The approach is sensitive to changes in collagen that are seen when malignancy develops and can also detect areas of necrosis that indicate underlying abnormality in the tissue not otherwise detectable by the surgeon.9,10
Photoacoustic imaging (PAI) is an emerging modality for biomedical imaging; its advantage is the ability to provide optical absorption contrast at ultrasound resolution.11, 12 It uses an ultrasound transducer to measure the ultrasonic waves generated from thermoelastic expansion resulting from a transient temperature rise due to the short pulse light absorption of biological tissue. The acquired ultrasonic waves are used to reconstruct the light absorption distribution which directly relates to vasculature of tumors or tumor angiogenesis—a fundamental step in tumor growth and metastasis.13, 14 In addition, if two optical wavelengths are used, the measured photoacoustic signals can be used to reconstruct the distribution of tumor oxygenation, which is an important indicator of tumor metabolism and therapeutic response.
Pulse-echo US, a conventional modality, can be readily achieved with a PAI system and provides tissue structure information at deeper depth than OCT, with resolutions that are scalable with the transducer frequency and bandwidth. Our group has investigated co-registered ultrasound and PAI for noninvasive transvaginal imaging for ovarian cancer detection.15
Combining these three approaches provides complementary information on optical absorption and scattering in tissue, as well as deep tissue structures. Other groups have developed systems that apply one or two of these modalities; for instance, for photoacoustic endoscopy, integrated intravascular OCT and ultrasound imaging, US-guided spectroscopic intravascular photoacoustic imaging, and for integrated OCT and photoacoustic microscopy.16-21 Some implementations, however, are not suitable for intraoperative use.
The imaging system
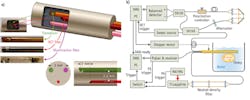
The system's multi-modality, 0.5-mm-diameter intraoperative probe consists of three components fixed inside a homemade structure: A ball-lensed OCT fiber (WT&T Inc., Canada); a multimode fiber (OFS Corp., CT) whose distal end is polished at a 45° angle for delivering the laser beam for PAI; and a high-frequency, unfocused US transducer. The square aperture of the ultrasound transducer is 0.5 × 0.5 mm. We chose a 35 MHz center frequency as a compromise between the axial resolution and penetration depth. The tip of the illumination fiber and the transducer element are aligned side-by-side with a 3 mm center-to-center separation. The OCT fiber is about 2.6 mm under the other two components and indents 2 mm towards the proximal end. Light exiting the illumination fiber is directed towards the imaging medium about 4 mm away from the center of the probe (see Fig. 1).
The Fourier domain OCT portion of the system is based on a 110 nm bandwidth swept source (HSL-2000; Santec Corp., Japan) with a center wavelength of 1310 nm and scan rate of 20 kHz. The source's 10 mW output power is split evenly into reference and sample arms by a 2 × 2 coupler. The side-view ball-lensed catheter collects the backscattered light and recombines it with the reference light using a second 2 × 2 coupler. A balanced detector (Thorlabs' PDB120C; Newton, NJ) detects the formed interferogram, while a 50 MHz digitizer (Gage Applied Technologies' Cs8325; Lockport, IL) acquires it with help from a 20 MHz anti-alias filter. The OCT probe is mechanically scanned laterally in steps of 3 μm over a 15 mm span to form the imagery. During OCT image processing, we apply wavelength-dependent amplitude correction to the raw data to correct for the optical power variation during A-line scans.22 The scaled measurement data is interpolated to a uniform grid in k-space before Fourier transformation.
The PAI system consists of a Ti:sapphire laser (Symphotic TII's LS-2134; Camarillo, CA) with a 700–950 nm tunable wavelength range, and is pumped by a Q-switched Nd:YAG laser (Symphotic TII's LS-2122). A neutral density filter attenuates the 20 ns, 15 Hz repetition rate output pulses, which are then coupled into the illumination fiber using a convex lens. The optical energy used in the experiments was about 1 mJ/pulse at a wavelength of 740 nm. The high-frequency transducer was connected to a Panametrics 5900PR (Olympus NDT Corp., Waltham, MA) ultrasound receiver, which was configured at 54 dB gain and with a 3–50 MHz bandpass filter. The laser triggers a 100 MHz digitizer (Cs12100, Gage Applied) with a 12 bit vertical resolution to acquire the amplified signal. The US system uses this same data acquisition setup—but uses a Panametrics 5900PR (which is also an ultrasound pulser) to do the triggering. A switch enables seamless switching between the PAI and US imaging modes.
In both US and PAI modes, the probe is mechanically scanned laterally in steps of 12.5 μm over a 15 mm span to form an image. We were able to improve lateral resolution using a synthetic aperture focusing technique (SAFT) and coherence factor weighting (CFW): The SAFT synthesizes a larger aperture by properly delaying and summing the measured signals from adjacent locations, while the CFW makes further improvements by compensating each image point with a ratio of the coherent versus incoherent SAFT sums.23
In our proof-of-concept study, the three imaging modalities acquired the images sequentially because PAI and US modes used the same data acquisition system, and the water coupling used for the ultrasound transducer prevented light from the OCT probe from reaching the intended spot because of the refractive-index matching. This limitation can be overcome, however, by coating the angled face of the OCT ball lens with a reflective material to reflect the light even in the presence of index-matching medium. The application of this technique would enable the acquisition of both OCT and PA images simultaneously. Currently, the repetition rate of the laser used for PAI is 15 Hz, which limits the data acquisition speed. This will be improved by using a higher-repetition frequency laser. The prototype endoscopic probe used a multimode fiber for PAI illumination; however, this fiber could be removed, and the illumination function transferred to the single-mode OCT fiber. The latter fiber in this case will need to have a damage threshold high enough to withstand the high laser energy. The removal of the extra multimode fiber would make it more convenient to assemble the probe and greatly reduce the probe size as well.
Characterizing tissue
We tested the performance of the system in ovarian tissue characterization using ex-vivo porcine and human ovaries. After imaging, the ovaries were fixed in formalin for histological processing and evaluation.
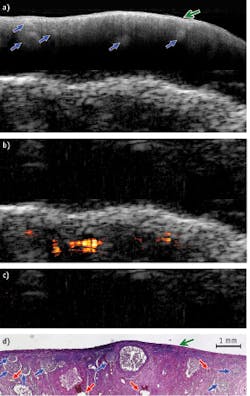
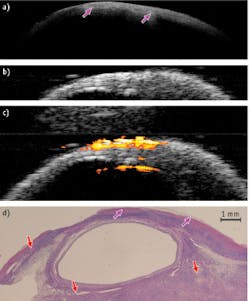
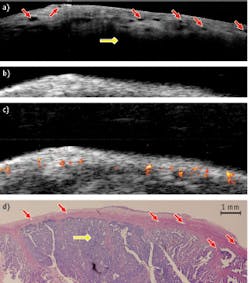
A healthy porcine ovary depicted by OCT, US, and PAI superimposed on US—and corresponding H&E stained histology images—demonstrate clearly that OCT and US complement each other: OCT offers higher resolution and more detailed structures at 1–2 mm under the surface, while US provides deeper structural information. Meanwhile, PAI identifies several vessels that are deeper than what OCT is able to detect (see Fig. 2).These results show that the hybrid device has a potential in ovarian cancer detection and characterization—and that there is great synergy in the combination of intraoperative or endoscopy modes.
ACKNOWLEDGMENTS
We thank OFS for supplying the illumination fiber for PAI, and Xiaohong Wang and Tim Greenwood at Anatomic Pathology Department of the University of Connecticut Health Center for helping with the tissue samples. This research was partly supported by the Connecticut Department of Public Health, NIH P41-EB2182, NIH R01CA151570, and the Donaghue Foundation.
REFERENCES
1. T. R. Rebbeck et al., N. Engl. J. Med., 346, 21, 1616–1622 (2002).
2. N. D. Kauff, et al., N. Engl. J. Med., 346, 21, 1609–1615 (2002).
3. W. A. Rocca et al., Lancet Oncol., 7, 10, 821–828 (2006).
4. J. S. Berek et al., Obstet. Gynecol., 116, 3, 733–743 (2010).
5. D. Huang et al., Sci., 254, 5035, 1178–1181 (1991).
6. E. M. Kanter et al., J. Biomed. Opt., 11, 4, 041123 (2006).
7. L. P. Hariri et al., Gynecol. Oncol. 114, 2, 188–194 (2009).
8. C. L. Evans, I. Rizvi, T. Hasan, and J. F. de Boer, Opt. Exp., 17, 11, 8892–8906 (2009).
9. Y. Yang, et al., Biomed. Opt. Exp., 2, 7, 1918–1930 (2011).
10. M. A. Brewer et al., Technol. Cancer Res. Treat., 3, 6, 617–627 (2004).
11. V. G. Andreev et al., "Opto-acoustic tomography of breast cancer with arc-array-transducer," Proc. SPIE, 3916, 36–47 (2000).
12. X. Wang et al., Nat. Biotechnol., 21, 7, 803–806 (2003).
13. N. Weidner, J. P. Semple, W. R. Welch, and J. Folkman, N. Engl. J. Med., 324, 1, 1–8 (1991).
14. P. Vaupel, F. Kallinowski, and P. Okunieff, Cancer Res., 49, 23, 6449–6465 (1989).
15. A. Aguirre et al., Transl. Oncol., 4, 1, 29-37 (2011).
16. J.-M. Yang et al., Opt. Lett., 34, 10, 1591–1593 (2009).
17. X. Li et al., Appl. Phys. Lett., 97, 13, 133702 (2010).
18. J. Yin et al., J. Biomed. Opt., 15, 1, 010512 (2010).
19. B. Wang et al., Opt. Exp., 18, 5, 4889–4897 (2010).
20. L. Li, K. Maslov, G. Ku, and L. V. Wang, Opt. Exp., 17, 19, 16450–16455 (2009).
21. S. Jiao et al., Opt. Exp., 18, 4, 3967–3972 (2010).
22. J. Gamelin et al., Opt. Exp., 17, 9, 7245–7258 (2009).
23. C.-K. Liao, M.-L. Li, and P.-C. Li, Opt. Lett., 29, 21, 2506–2508 (2004).
Yi Yang and Tianheng Wang are graduate students at the University of Connecticut, Dept. of Electrical and Computer Engineering (Storrs, CT), and Xiang Li is a graduate student at the University of Southern California, Dept. of Biomedical Engineering (Los Angeles, CA). Contact Professor Quing Zhu at [email protected].