NATIONAL INSTITUTES IMPACT REPORT: Funding for OCT: Technologies, applications and businesses
The foundation for today's multi-billion dollar optical coherence tomography (OCT) market was established during the late 1980s and early 1990s, when the National Institute of General Medical Sciences (NIGMS) funded research at the Massachusetts Institute of Technology (MIT; Cambridge, MA) on femtosecond laser tissue interaction. Over the years, NIH has continued to support OCT across a range of institutes, including the National Eye Institute (NEI), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the National Heart Lung and Blood Institute (NHLBI), National Arthritis and Musculoskeletal and Skin Diseases, the National Cancer Institute (NCI) and the National Center for Research Resources.
The first clinical application of OCT—ophthalmology, established in the 1990s—did not by itself spawn commercial and clinical success in new application areas, however. "By 1994, little or no work was being done on anything but the eye," explains OCT pioneer Mark Brezinski, director of the Optical Coherence Tomography Laboratory, Brigham and Women's Hospital (Boston, MA). A grant from NHLBI enabled Brezinski to further his research into cardiovascular applications and demonstrate that OCT could image nontransparent tissue.
The expansion of OCT beyond ophthalmology into areas such as cardiology, orthopedics and gynecology was a direct result of NIH support, says Brezinski. "The technology may have taken another decade [to become a clinical tool] without NIH funding." NIH support of OCT in the mid-1990s opened the door for applications such as assessing vulnerable plaques within blood vessels; collagen levels in tendons during rotator cuff surgery; and changes to the cervix. NIH continues to fund ophthalmology applications such as early detection and assessment of glaucoma and diabetic retinopathy. In the early days of OCT, grant applications were heavy on technology development and light on applications. Today, that trend has reversed and the majority of grants focus on applications. However, important technology developments such as new laser sources continue to receive support (see Fig. 1).
Along the way, NIH program officers have encouraged researchers and recently small businesses to pursue funding for OCT. Long-time OCT researchers say that these individuals championed OCT when the technology was on the verge of becoming forgotten. "They knew where they wanted to be long-term," says Brezinski. "They stayed true to their long-term goals, but remained flexible in their approach." This support, coupled with the elegance of OCT's technical approach, has led to one of the fastest growing imaging modalities today.
From research to business
NIH funding of OCT has grown from roughly $5 million in 2000 to over $60 million in 2010. "The trend is going up with more application-oriented OCT projects supported," says Yantian Zhang, who oversees NIBIB's optical and molecular imaging program. NIH will continue to fund OCT through research grants; however, the institutes are also strengthening their small business funding mechanisms, which will provide additional opportunities for start-ups interested in commercializing OCT systems and devices.
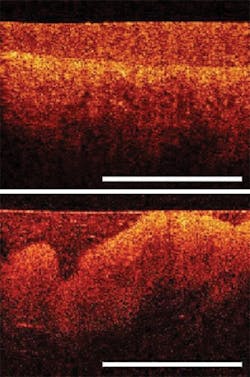
Since the budget for NIH's Small Business Innovation Research (SBIR) program is 2.5% of the entire NIH budget, the amount of money set aside for small business development is substantial. In 2010, the set aside was $616 million. In the last four years, NIH has funded an average of 13 OCT SBIR projects each year. These numbers are expected to rise as more companies enter the OCT commercial space. In particular, applications of OCT to cancer diagnostics and treatment are viewed as a growth area (see Fig. 2). "There is a very clear interest in OCT in cancer, which is new," says NCI SBIR program officer David Beylin. "Companies that will play a major role in cancer are just being formed. SBIR will play an increasing role in showing the effectiveness of OCT in cancer."Although obtaining SBIR money can take about 9 to 12 months from start to finish and intellectual property becomes public domain, many start-up OCT companies are interested in tapping into SBIR funding. The government is an attractive financing option for a small company since their financing choices are limited, says Andrew Cittadine, a consultant with Diagnostics Photonics (Chicago, IL). Venture capital and corporate partnerships are key investment sources, but for early stage development, SBIR funding can stretch those investment dollars and validate the technology through the SBIR peer-review process.
"There is a tipping point when a technology becomes attractive enough to attract outside investments. We're getting close to that now with OCT, and government funding has helped get to that point," Cittadine says.
To make the best use of small business grant dollars, some institutes are retooling their SBIR programs. Rather than spread their SBIR grants among program areas as many institutes do, the NCI recently consolidated all of their SBIR efforts into a single program. Each program officer has expertise in a core technical area, as well as industrial and small business experience. "We work closely with companies in our portfolio not just to fund them, but to help them commercialize their technology," says Beylin.
Benefits of public/private partnerships
NCI has developed two programs that help build public/private partnerships, and OCT is benefiting from both. The first, NCI's Investor Forum, provides an opportunity for a select group of SBIR-funded companies to network with private investors. At the second annual forum, held in November 2010, over 100 venture capital firms and strategic partners met with 14 pre-selected companies that have received NCI SBIR funding. Among those companies was Imalux (Cleveland, OH), developer of the Niris Imaging System, an OCT system to detect early stage cancers. Forty Niris units have been placed in major academic centers around the world and over 1,700 patients have been treated with the system. Because Niris received broad-based clearance from the U.S. Food and Drug Administration to scan throughout the body, it has the potential to be used in an array of applications. A second-generation time-domain Niris system will be introduced in the first quarter of 2011.
Another NCI public/private funding mechanism is the SBIR Phase II Bridge Awards. Now in its second year, this option provides up to $1 million a year for three years for previously funded SBIR Phase II projects. The award aims to bridge the gap between the end of Phase II funding and the next round of private financing needed to fully commercialize a product. It also requires recipients to secure matching funds from outside sources. Currently, 10% of the NCI Bridge portfolio supports OCT efforts.
Praevium Research (Santa Barbara, CA), developer of tunable laser sources, received a 2010 Bridge award to develop a new OCT swept laser source based on VCSEL technology (see Fig. 3). Praevium is partnering with Thorlabs (Newton, NJ) and the OCT group at MIT. "Cancer is still an emerging application area for OCT and has huge potential," says Vijay Jayaraman, Praevium's founder and CEO. To take advantage of that potential, OCT must be able to perform volumetric tissue imaging, but currently available sources aren't up to the task. "OCT has been at the mercy of telecom lasers," says Jayaraman. A new VCSEL source will allow for rapid tuning over a wide range of wavelengths, with better resolution at greater tissue depths.NHLBI, another key OCT funder, has also analyzed its SBIR efforts and is awaiting a new director before implementing recommendations to consolidate SBIR programs. The institute recently established a Phase II Competing Renewal Award, which is similar to NCI's Bridge Award, to help companies continue to innovate while they look for private investment partners.
OCT as economic driver
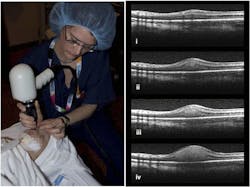
As OCT matures, small businesses willing to explore new clinical applications and designs will drive innovation. "By finding ways to make OCT cheaper and more user-friendly, it [OCT] can become an important screening tool," says NEI Program Officer Jerome Wujek. OCT's ability to image changes in tissue at the earliest stages of disease development will help clinicians devise appropriate treatment plans. "OCT will be as ubiquitous as ultrasound," says Eric Buckland, CEO of Bioptigen (Durham, NC), which creates imaging systems based on spectral-domain OCT (SD-OCT) technology. SBIR funding helped Bioptigen develop the first handheld OCT imaging device, which is now used to image retinal development in pre-term infants (see Fig. 4). Aligning an eye with a 3 lb. scanning head rather than cobbling together an 80 lb. system has opened up OCT surgical applications, Buckland says.Currently, no SD-OCT products target the surgical market, but Buckland says that SBIR funding is helping the company develop a high-speed volumetric imaging system with resolutions better than the 5 to 10 μm currently available on commercial systems. Because the SD-OCT system allows surgeons to refine repairs while patients are still in surgery, fewer follow-up surgeries are needed.
Over two decades, NIH's funding of OCT through research grants and now SBIR grants has created an important research and clinical imaging tool. But as Brezinski maintains, NIH's support of OCT has also created jobs. He cites statistics that for every one high-tech product job, nine others are created to support that job. "When funding is cut to NIH, long-term productivity is cut down the line," he says. "If you take away NIH, then you take away the competitive advantage for the U.S. economy."
About the Author