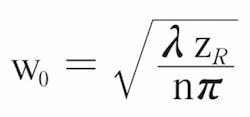Multiple beams boost resolution of OCT
The freqency-domain approach broke the first significant barrier to optical coherence tomography’s potential. Now a multibeam approach promises similar improvement, as an oral-cancer study demonstrates.
By Gordon McKenzie, Waseem Jerjes, Zaid Hamdoon, and Colin Hopper
Optical coherence tomography (OCT), a powerful cross-sectional modality allowing noninvasive imaging of samples in real time and at high resolution, has become the new gold standard in ophthalmic care. But while it is extremely promising for other clinical applications, its use in those areas is not widespread. That is due at least in part to technical constraints, which equipment makers are addressing. One approach, multibeam OCT, promises to advance the use of OCT in cancer detection.
OCT was conceived independently by Tanno and Chiba of Yamagata University in 1990, and by Huang and Fujimoto at MIT in 1991.1, 2 It is normally based around a Michelson interferometer, an invention dating back to 1887.3 The technology uses a coherence gate to spatially locate a reflected signal from within a sample. An optical focus provides lateral resolution, and beam-scanning enables 2-D and 3-D scans.
Early OCT systems operated in the time domain. Improvements such as broad-band light sources and dynamic focusing supported its development, but first-generation OCT had a significant limitation: speed. The scanning mechanism’s mechanical limitations meant image capture would take multiple seconds–too slow for use in vivo. Fercher’s group overcame that bottleneck in the mid 1990s by collecting the interferogram in the frequency domain (FD-OCT), which gave a signal-to-noise advantage of about three orders of magnitude.4, 5 Advances in light sources and detectors since then have enabled very rapid image capture.
But even FD-OCT has a significant disadvantage: it is not possible to achieve high lateral resolutions by dynamic focusing because the method involves collection of light from all depths simultaneously (followed by a Fourier transform to recover the depth data). While axial resolution is determined by light source properties, the lateral resolution is a function of the numerical aperture (N.A.), which is a function of the desired depth of focus.
The Gaussian beam waist radius w0 is related to the depth of focus ZR (using the well-known Rayleigh criteria) by the following formula:
At the usual wavelength of 1300 nm used for imaging tissue (n ≈ 1.35), a 1 mm depth of focus implies a minimum Gaussian beam radius of 17.5 µm (equivalent to 20.6 µm FWHM). This is often too large for useful clinical images.
Multibeam OCT
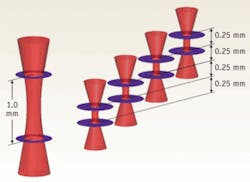
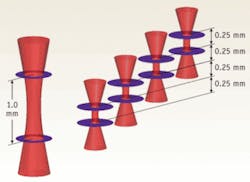
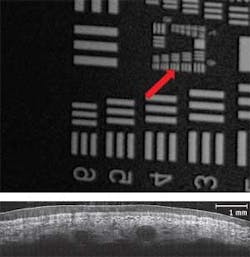
To overcome this fundamental limitation of traditional, single-beam FD-OCT systems, Michelson Diagnostics has developed a solution to simultaneously scan multiple beams, focused at slightly different depths, and compile an image mosaic from the resulting multiple FD-OCT interferograms. Using a multichannel interferometer, the beams are sufficiently close together to be effectively simultaneous, so motion artifacts are not a problem. For example, a four-beam system focused over 1.0 mm, with each sub-beam focused over 0.25 mm, produces double the resolution possible from a single beam (see Fig. 1). Each sub-beam has a theoretical FWHM diameter of just 10.3 µm.
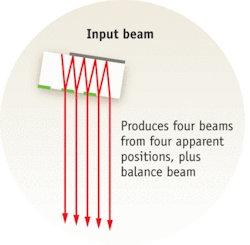
Michelson Diagnostics first implemented the multibeam OCT approach in its EX1301 OCT Microscope (see Fig. 2). This microscope is based on a free-space Michelson interferometer, using a “rattle plate” to generate a series of virtual sources at the input to the interferometer. The instrument achieves the required lateral resolution of less than 10 µm (see Fig. 3); blending algorithms eliminates minor mismatch of intensity across the mosaic-tile boundaries.
Another way of generating multibeam OCT is to use a probe with multiple fibers. This approach was pioneered by Victor Yang et al. at Ontario Cancer Institute, Princess Margaret Hospital, University Health Network (Toronto, Canada). A collaboration between Michelson Diagnostics and UHN Toronto has demonstrated successful in vivo imaging of a lapine colon with a prototype four-beam fiber-optic probe (see Fig. 4).
Oral cancer: the case for OCT
OCT has the potential to greatly improve the effectiveness of detection and management of oral cancer, the 11th most common cancer worldwide. World Health Organization figures show 405,000 new cases annually, with an upward trend. The five-year survival rate, approximately 50%, has not changed significantly in 30 years despite the fact that cancers detected early are much more survivable. The usual treatment is surgical removal of the tumor; early detection enables less invasive surgery with greatly reduced effects on appearance as well as on eating and speaking functions. Late detection requires more expensive, invasive and disfiguring surgery, and requires costly aftercare.
After a patient with oral cancer is treated, continuous monitoring is necessary to ensure recurrence is picked up and treated at an early stage. A delay in diagnosis can lead to the spread of new disease, increasing morbidity and mortality. The current state of the art for monitoring lesions is very far from ideal: it requires a combination of objective (clinical examination, including visual inspection) and subjective (surgical biopsy and histopathology processing) techniques.
A thorough surveillance program would be unacceptably painful, stressful, and damaging to the patient’s oral tissues, with biopsies impacting both aesthetic and functional structures (multiple biopsies can cause fibrosis). OCT, with its ability to precisely monitor changes in tissue architecture, could regularly and noninvasively alert the clinical team to any changes needing further attention (for example, a more senior opinion or a surgical biopsy).
Standard diagnosis and treatment
A patient suspected of oral cancer today is typically referred to a specialist clinic by a doctor or dentist who notices initial symptoms such as nonhealing red (erythroplakia), white (leukoplakia) or mixed (speckled erythroplakia) lesions. The first job of the clinical specialist is to try to eliminate the worried well patients from those who are of clinical concern.
A patient judged to be of clinical concern must undergo surgical biopsy, and will have to return to the clinic one to two weeks later to receive the results. If the diagnosis shows oral dysplasia/cancer, further treatment and/or follow-up will be advised. The patient will typically fall into one of three groups.
If the patient’s pathology is benign or mildly dysplastic, he/she will not be treated, but will be recalled to the clinic for monitoring every few months. This process will last for years and can involve further painful and destructive biopsies.
If the patient has a moderate or severe dysplasia or a combination (for example, mild to moderate, moderate to severe), he/she will require immediate treatment. The lesion will be treated using a variety of methods, which may include surgical excision, CO2 laser excision, ablation, or photodynamic therapy (PDT). Once the treatment has been completed, these patients will also be put into a monitoring cycle, attending the clinic every month initially, then every three to six months. The clinical specialist will give advice on risk factors and self-examination. Most of the patients in this group end up being reviewed annually for life; surgical biopsy may be acquired many times.
A final group will be found to have invasive cancer and require a more complex treatment process, which is, frankly, beyond the scope of this article.
Where OCT adds most value
Histopathology is severely lacking in its ability to predict or observe progression, and it provides only a snapshot of a part of the lesion, destroying the tissue and the pathology in the process. Clearly, there is a pressing need for a tool that allows accurate monitoring of oral tissue lesions for the first two groups of patients. OCT is noninvasive, nondestructive, can be used to systematically scan the whole lesion, and is able to quantify tissue architecture in terms of depth and size. It is ideal for monitoring change in patients in the first two groups. Even for patients in the third group, OCT can be of major benefit.
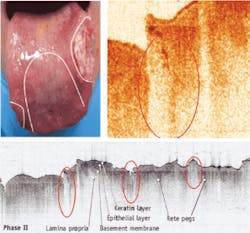
A recent study carried out at the National Medical Laser Centre, University College London and the Head and Neck Unit, University College Hospital (England), compared the potential of the multibeam Michelson Diagnostics EX1301 OCT microscope with histopathology.5 The comparison involved 24 suspicious oral lesions (from 19 patients) that were excised and scanned. The study’s key conclusions were that OCT can serve effectively as an adjunct to clinical examination. The OCT scanner can be used by the examining clinical specialist to assess the need for surgical biopsy, in vivo and in real time. The clinical specialist can examine the OCT image for worrying signs including thinning and thickening of the keratin layer, thinning (atrophy) and thickening (acanthosis) of the epithelial layer, thickening (breach) of the basement membrane, and thickening (corium) of the submucosal layers, plus tumor cell aggregation (crowding) and pattern of invasion.
The study provides the basis for recognizing four major initial opportunities for multibeam OCT to assist in the clinical pathway.
During the initial clinical (visual) exam, multibeam OCT can be used to help exclude the worried well from the clinical process as quickly and safely as possible, by providing more information than is currently available to the clinician from visual examination.
During follow-ups, multibeam OCT can enable quantitative analysis of disease progression. The ability of OCT to record the thickness, architectural changes of specific tissue layers, and breach of the basement membrane is the key to allowing accurate measurement of progression.
Multibeam OCT can provide out-patient assessment of precancerous (dysplastic) oral lesions. Initially this is likely to be for localizing and guiding biopsy sites for nonhomogenous large lesions. This capability has the dual benefit of reducing the flow of healthy tissue into the histopathology process, and ensuring that the most relevant samples are taken quickly and analyzed quickly.
During treatment, to image tissue structure changes due to cancer, to enable detection of early-stage cancers, avoiding major ablative surgery and chemoradiation. It is possible that OCT could reduce oral cancer deaths.
A powerful and flexible imaging tool, multibeam OCT overcomes resolution limitations of FD-OCT. with the ability to provide more detailed imaging of tissue architecture and pathologies and better contrast, it has significant near-term potential to address such pressing clinical needs as early detection of oral cancer.
References
- N. Tanno, T. Ichikawa & A. Saeki (1990). “Lightwave Reflection Measurement,” Patent JP2010042
- D. Huang et al., (1991) Science 254(5035), 1178 (1991).
- http://en.wikipedia.org/wiki/michelson_interferometer
- A.F. Fercher, C.K. Hitzenberger, G. Kamp and S.Y. El-Zaiat Optics Communications 117(1) 43 (1995).
- A.F. Fercher, W. Drexler, C.K. Hitzenberger & G. Kamp (1994) “Measurement of optical distances by optical spectrum modulation” Ed. A.F. Fercher et al. Vol. 2083, 263. Presented at Microscopy, Holography, and Interferometry in Biomedicine, SPIE, Budapest, Hungary (1994).
- W. Jerjes et al., British J. of Oral & Maxillofacial Surgery, In Press.
Dr. Gordon McKenzie is cofounder and medical director of Michelson Diagnostics, 11A Grays Farm Production Village, Orpington, Kent, UK, www.md-ltd.co.uk. Waseem Jerjes is a senior research fellow in surgery, and Zaid Hamdoon is clinical assistant, both working with Colin Hopper, a senior lecturer/consultant maxillofacial/head and neck surgeon at University College Hospital, London. Dr. McKenzie can be reached at [email protected].