Complex monolithic optics enrich fluorescence imaging
Microscopes incorporating CMO design are smaller, lighter, more robust, and more reliable.
By Alan C. Graham and S. Jeffrey Rosner
Fluorescence techniques are widely used in a variety of biological research applications, pharmaceutical discovery processes, and clinical diagnostics. Alongside this growth in technique has been a parallel development of both fluorescent reagents and optical instrumentation. The majority of the measurements using fluorophores are designed around identifying and localizing single molecules and identifying structures within single cells for such applications as:
Biophysics. Many of the current fluorescence techniques were borne out of early biophysical experiments focused on localizing DNA, peptides, and proteins within cells. These experiments have evolved from directly identifying and localizing specific biomolecular interactions to the use of fluorescent tagging as an aid in measuring kinetics, thermodynamics, and bonding strength of protein-protein, protein-DNA, protein-RNA interactions, DNA hybridization, and protein folding.
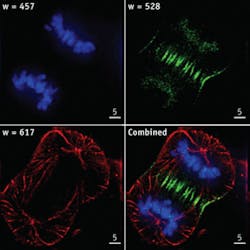
Cell biology. Fluorescence techniques have been used to illuminate cell structures (such as the cytoskeleton and nucleus), organelles (such as the endoplasmic reticulum, mitochondria, and golgi), and proteins in the cytoplasm and cell membrane. In addition to identification and navigation, these techniques have allowed the localization of various biomolecular phenomena (see Fig. 1).
Neuroscience. Ca+ imaging has been used to time-resolve signaling processes in neural structures.
Systems biology. Using increasingly sophisticated techniques that localize and specify molecular interactions, accurate determination of biomolecular interactions and protein expression have been quantified with increasing precision.
Reagent development has largely followed the development of illumination sources, with absorbance spectra carefully tuned to available laser wavelengths. Initially these were designed around argon and HeNe laser lines, which were the most convenient sources available during the early phase of this development. Fluorophores designed for covalent attachment to biomolecular chemistries have been commercialized at these available wavelengths, with substantial Stokes shifts of the fluorescence allowing rejection of the illumination with straightforward optical systems.
More recently the widespread availability of solid-state lasers has enabled the proliferation of many wavelengths; however, this trend also increased the complexity of the illumination and detection systems. As a result, monochromator systems evolved to provide wavelength flexibility. In addition, laser combiners using discrete optics and up to four lasers have emerged, but they are expensive, cumbersome, and difficult to maintain in alignment.
In addition to advances in these technologies, development of microscopy techniques has accelerated, with a variety of sophisticated, high-resolution imaging schemes that utilize multiple fluorophores to carefully identify and locate biochemical structures, molecules, and molecular interactions. While a full discussion is beyond the scope of this article, there is a rich literature base discussing techniques such as total-internal-reflection fluorescence, fluorescence resonant-energy transfer, fluorescence lifetime imaging, fluorescence recovery after photobleaching, and fluorescence in situ hybridization. All of these approaches use multiwavelength illumination to provide high specificity to a mixture of fluorophores used in various assays. An excellent tutorial is available at www.microscopyu.com/articles/fluorescence/index.html.
In cytometry, similar tagging of biomolecules is used to label cells in highly specific ways. While imaging is rarely used in these systems, the illumination and detection of fluorescence techniques used in these systems is quite similar to that used in microscopy systems. A state-of-the-art cytometry system is well-described at www.bdbiosciences.com/immunocytometry_systems/products/display_product.php?keyID=53.
Traditional illumination solutions
White-light monochromators have emerged as a straightforward approach to multiwavelength illumination for excitation of fluorescent dyes and proteins in microscopy and cytometry applications. However, only a small percentage of light from a white-light source is available for any particular wavelength or application, and light at other wavelengths can reduce system contrast and decrease the signal-to-noise ratio. Researchers are thus looking to combinations of discrete-wavelength sources (lasers and LEDs) to replace white-light monochromators.
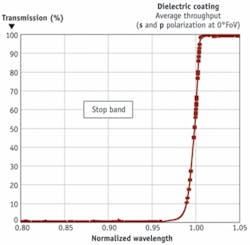
Whereas white-light monochromators require light separation, discrete-wavelength sources require light combination. Evaporated or sputtered dichroic thin films, a common method of combining (or separating) two discrete wavelength sources, act as filters that selectively transmit light of one spectral region (pass band) and reflect another (stop band) regardless of polarization or magnitude of spectral separation (see Fig. 2). Polarization-preserving coatings that function at closely spaced spectral bands, however, are more complex.
Plate dichroic filters (beamsplitters/combiners) can be constructed of alternating layers of high-index films such as titanium dioxide and low-index films such as silicon dioxide, with the average layer thickness on the order of a quarter of the stop-band wavelength. The low refractive index of the incident medium (n of approximately 1.0) makes these coatings relatively straightforward to design. Generally, the dichroic interface is oriented at a 45° relative to the light path, and precise optomechanical hardware is required to mount and align the filters.
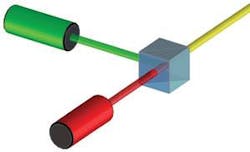
Dichroic filters can also be immersed between two right-angle prisms (see Fig. 3). However, the n∙sin (θ) relationship between the incident medium and the film layers shows that light propagates at larger effective angles in the thin-film layers, resulting in a more dramatic separation of the orthogonal polarizations. This effect complicates the thin-film design, especially when the coating is required to be polarization insensitive. And although the normal incidence of a cube configuration simplifies the mounting and alignment requirements, the prisms must be fabricated to exacting tolerances or precise tooling must be used to integrate the cube with other optical and optomechanical assemblies.
Optomechanical assemblies typically evolve from a series of discrete, free-space optics with epoxy and metallic structures used to connect and align them. Optomechanical beam combiners multiplex numerous illumination sources using combinations of discrete dichroic filters. Generally, N discrete illumination sources require N–1 components with N–1 dichroic interfaces (see Fig. 4). Even with moderate numbers of sources, this type of assembly is inherently high-loss due to the number of interfaces that the beams propagate through. The light source farthest from the output of a stack of dichroic filters immersed between glass prisms has a throughput efficiency T, given by:
T = (1–S1)∙(1–S2)∙(1–Bn)N–2∙(1–Dn)N–1where S1 is the entrance aperture loss (~0.25% reflection loss for single-wavelength AR); S2 is the exit aperture loss (~0.50% reflection loss for broadband AR); Bn is the interoptic interface loss (~0.5%); and Dn is the dichroic interface loss.
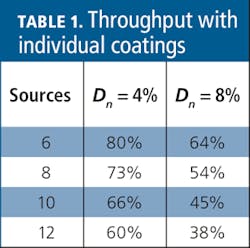
Although absorption and scatter losses can be minimized at each dichroic interface, a 4% to 8% loss per interface is not unreasonable for multichannel applications. As a result, the throughput of the farthest channel decreases rapidly with increasing channel count (see Table 1).
Transmission through a sequence of individually mounted plate beam-combiner coatings can be more efficient than immersed coatings, but time-dependent alignment drift, surface contamination, and scatter degrade the performance over time, resulting in performance comparable to the sequence of cubes.
In addition to low efficiency, optomechanical assemblies can be expensive to procure and maintain. The numerous optomechanical assemblies used to combine discrete optical components together tend to be costly, bulky, and massive, and require painstaking alignment in the factory. These systems also can require periodic field alignment to maintain precision, so fiber-coupling architectures are often used, sacrificing optical precision and flexibility.
Complex monolithic optics
Monolithic integration of optical components is a familiar concept in multielement lens manufacturing, in which lens doublets are an example of the innate advantages of monolithic assembly. Optomechanically assembled doublets consist of two lenses separated by a tubular housing and threaded placement rings, with surfaces that are precision-machined to match the optical surfaces. Not only is the optomechanical assembly expensive, but it is subject to long-term drift and hysteresis. In contrast, the now ubiquitous monolithic doublet is more cost effective and more environmentally robust.
Complex monolithic optics (CMOs) are a recent monolithic design paradigm for planar optics in which discrete optical functionalities such as chromatic separation, chromatic combination, beam steering, amplitude control, and phase control are integrated together without reliance on numerous mechanical assemblies. In essence, CMOs are single, prealigned structures like their multielement lens counterpart, with all alignment tolerances designed into the manufacturing process eliminating the requirement for initial alignment. Because the alignment is an integral part of the CMO assembly, it does not drift with vibration, environment, or age.
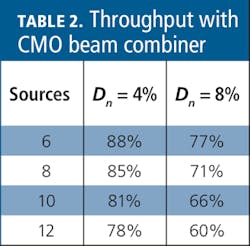
Rhomboids are a simple CMO analog to the multielement lens. Used to combine N discrete sources, CMO rhomboid beam combiners can precisely control beam paths, enabling thin-film coating designs that are not practical with non-CMO assemblies. Agilent’s patent-pending CMO beam combiners reduce the number of interfaces in the farthest channel to N/2 dichroic interfaces plus the entrance and exit apertures, resulting in a significantly improved transmission (see Table 2).
The transmission of a six-source CMO combiner is nearly 10% higher than an optomechanical assembly and nearly twice that with larger numbers of sources.
CMO assemblies for microscopy
Numerous discrete-wavelength sources with the performance and control characteristics required for a wide variety of fluorescence applications in the life sciences are commercially available. Eight-channel CMO beam combiners that multiplex common laser and LED wavelengths are being introduced in the market in the first half of 2008. Further, because the transmission efficiency of CMO combiners scales favorably with increased channel counts, beam combiners with higher channel counts can be readily manufactured.
Later this year, CMO technology will address compact beam separators that will be available at a variety of wavelengths with output beams for multichannel fluorescence imaging and cytometry applications. Together, CMO beam combiners and CMO beam separators will enable microscopy tools to be smaller, lighter, more robust, and more reliable.
Alan C. Graham is business development manager, Agilent Precision Optics, Nano Positioning Metrology Division at Agilent Technologies and S. Jeffrey Rosner is the chief technology officer, Agilent Materials Science Solutions at Agilent Technologies (Santa Clara, CA). Contact Graham at [email protected].