Researchers to present optical techniques for counting single viruses outside the lab
Two separate teams of researchers have developed new optical methods for determining the exact viral load of a sample by counting individual virus particles. These new methods are faster and cheaper than standard tests and they offer the potential to conduct the measurements in a medical office or hospital instead of a laboratory. The teams will present their latest results at the Conference on Lasers and Electro-Optics (CLEO: 2013; June 9-14, San Jose, CA).
One research group, led by electrical engineer and bioengineer Aydogan Ozcan of the University of California, Los Angeles (UCLA), is working to directly image single virus particles using holographic microscopy. The other, led by electrical engineer Holger Schmidt of the University of California, Santa Cruz (UCSC), is detecting single particles tagged with fluorescent labels on a microfluidic chip. Both teams expect to use their work to develop commercial instruments useful for on-site diagnosis and monitoring with rapid results and fast turnaround.
Related: Algorithms and cell phone add-on enable powerful, inexpensive microscopy
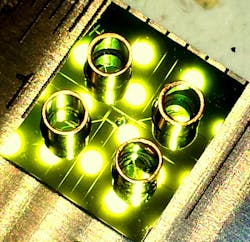
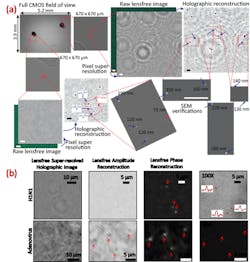
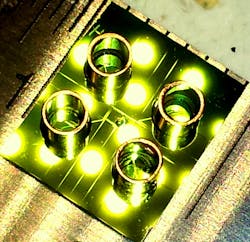
Ozcanâs UCLA team has demonstrated the ability to capture optical images of single viruses and nanoparticles over a comparatively large field of viewâabout the size of a postage stampâusing nanolenses that self-assemble around the virus particles like little magnifying glasses.
âBecause viruses are very smallâless than 100 billionths of a meterâcompared to the wavelength of light, conventional light microscopy has difficulty producing an image due to weak scattering of sub-wavelength particles,â Ozcan says. When lighted, the teamâs new nanolens-nanoparticle assembly projects a hologram that can be recorded using a CMOS imager chip (a type of semiconductor-based light detector) and digitally reconstructed to form an optical image of the particle. âThe resulting image improves the field of view of a conventional optical microscope by two orders of magnitude,â says Ozcan.
This wide field of view allows the device to form images of many nanoparticles in a single photograph and provides a high-throughput platform for a direct and accurate viral load count. The instrument can be made sufficiently compact and lightweight for field applications and, attached to a cell phone, could become useful even in remote locations.
The UCSC researchers will present the results of a collaborative effort between UCSC, Liquilume Diagnostics Inc. (San Jose, CA), and the groups of infectious disease clinician and virologist Charles Chiu at University of California, San Francisco, and engineer Aaron Hawkins at Brigham Young. While Ozcan's group visually counts individual viruses, Schmidtâs counts them by detecting their nucleic acidsâthe genetic makeup of the viruses. The nucleic acids are labeled with a fluorescent dye, and light from the fluorescence is detected as they pass through a channel in a microfluidic chip about the size of a thumbnail.
Current tests for determining viral load generally rely on a technique called polymerase chain reaction (PCR), which amplifies a small sample of nucleic acid, such as DNA, and makes it easier to detect. âThe gold standard for viral load detection is PCR, due to its sensitivity and specificity,â Schmidt says, but PCR is limited to merely estimating the number of viruses. In contrast, the new method counts real particles as they pass through the fluorescence detector on the chip. âWe have demonstrated actual virus counts of specific nucleic acids in less than 30 minutes with minimal sample workup,â Schmidt says. So far, the group has collected reliable data on samples diluted to a point well within the range required for clinical detection.
Unlike direct visualization techniques, Schmidt's chip-based method requires that the targeted virus particles be labeled. The labeling technique would allow clinicians to target specific viruses while ignoring unlabeled background material. This makes the process potentially useful in situations where clinicians already know what they are looking forâoften the case for viral load tests.
The chip is currently housed in an instrument about 1 ft2, making the device portable. Along with rapid analysis turnaround, this portability should make the technique useful for point-of-care tests. In addition to detecting viruses, the device may also find uses as a sensor for cancer biomarkers, for environmental analyses of chemicals, and even in industrial production monitoring.
CLEO: 2013 presentation AW1I.6. âHigh-throughput Imaging of Single Viruses using Self-assembled Nano-lenses and On-Chip Holographyâ by Aydogan Ozcan will take place Wednesday, June 12, at 12:15 p.m. in the San Jose Convention Center.
CLEO: 2013 presentation CM1M.7. âClinical Detection of Viral Infection on an Optofluidic Chipâ by Philip Measor will take place Monday, June 10, at 9:45 a.m. in the San Jose Convention Center.
-----
Follow us on Twitter, 'like' us on Facebook, and join our group on LinkedIn
Subscribe now to BioOptics World magazine; it's free!