A richer view of bio structures
JAMES BEACH
The value of hyperspectral microscopy for life scientists is the ability to acquire the optical spectrum of all points in a microscope image, coupled with specialized spectral analysis. The approach produces uniquely rich views of biological tissue, yielding revelations for both research and clinical applications. For instance, hyperspectral imaging (HSI) can distinguish normal, precancerous, and cancerous cervical cells on Pap-test slides based on the combination of their morphological and spectral characteristics, as a prelude to development of prescreening tests for more efficient cervical-cancer diagnosis.
Methodology and instrumentation
Hyperspectral microscopy grew out of two unrelated disciplines born in the 1970s and 1980s: microspectrophotometry and spectral remote sensing. The former is well known to cell researchers for following photochemical reactions and revealing properties of components within cells’ interior. The latter was a NASA creation for capturing and interpreting spectral information from the surfaces of distant targets like the Earth’s surface below an aircraft.
The disciplines began to merge when cell scientists turned to digital cameras and image-analysis software designed for image arrays. It became possible, with technologies like the filter wheel, to rapidly change wavelengths while taking a series of pictures to create spectral images. Electronically tuned liquid-crystal and acousto-optic filters increased the speed and number of wavelengths, and added programming flexibility to spectral sequences. As far back as the early 1980s, flat-field spectrographs using holographic gratings could faithfully reproduce the spectrum of all points along the spectrograph slit, creating one-dimensional spectral images. When the scene is scanned across the spectrograph slit, a two-dimensional hyperspectral image (a hypercube with two spatial dimensions and a third spectral dimension) is produced.
For microscopists, scanning was already in place with motorized stages. The remaining problem was to integrate all of the hardware and software components into a system for hyperspectral microscopy, and aim this at a market that would benefit from the power of the technology.
HSI for bio
In early 2008, CytoViva (Auburn, AL) saw the opportunity for commercial hyperspectral microscopy to serve the growing research in nanomedicine. The company worked with Headwall Photonics (Fitchburg, MA) to incorporate its VIS-to-near-infrared (IR) spectrographic camera to enable sensitivity over the visible and near-IR spectral ranges that have been reported for nanoparticle applications. ITT-VIS (Visual Information Solutions of Boulder, CO), developer of remote sensing software, supplied the sophisticated spectral-analysis features of its ENVI software. And Bruxton (Seattle, WA) provided its SIDX device interface product to add instrument controls, converting the file-input orientation of ENVI into an integral part of the product.
The group designed the hyperspectral imagery (HSI) microscope system to work with bright- and dark-field transmission modes, and with incident light for reflectance and epifluorescence. It includes the spectral detector, a second color camera, an automated stage, a halogen light source and dual monitors, which are integrated with a research microscope (Fig. 1). An optional live chamber mounts to the microscope stage so researchers can examine living cells in real time at high resolution. By November 2008, two CytoViva HSI microscopes were delivered to government research facilities at the FDA and USDA.
The hyperspectral microscope is designed to take full advantage of its high-intensity dark-field illuminator, giving researchers a much brighter view of nanoscale structures than is available with other methods. It provides annular structured illumination at a low angle of incidence onto the sample located just above the condenser. The resulting dark field of illumination is approximately 150 times brighter than is possible with conventional dark field, and can effectively scatter light from nanoparticles with enough brightness to enable the capture of spectral information. Virtually all the light that is collected has interacted multiple times with sample components and carries the unique spectral signatures of the sample constituents. The smooth spectral output from a halogen source is used to avoid problems with spectral analysis when line structure is present, as it is with commonly used mercury, xenon, and metal halide sources.
Visible and near-infrared wavelengths between 400 and 1000 nm are resolved with an imaging spectrograph containing an original holographic grating, and recorded at 12-bit depth. Hyperspectral images are produced by moving the target under the microscope objective to the position sampled by the spectrograph slit, and recording the spectra of all points along the line onto the digital camera. The target is then moved a very short distance using the automated stage (from Prior Scientific, Boston) to bring the adjacent region of the target to the recording position. The process repeats until the area of interest surrounding the target has been recorded. The resulting HSI data are represented as a three-dimensional structure that holds a stack of conventional two-dimensional images, each containing a narrow band of wavelengths that collectively cover the spectral range.
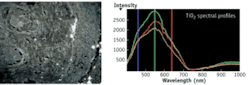
The result is the ability to acquire spectral information from samples in a way that allows one to separate the unique spectral features of a target molecule or other object from background spectra in a sample, or to unmix the spectral information from single pixels. Objects such as gold, silver, and TiO2 nanoparticles, carbon nanotubes, fluorescent probes, quantum dots, and many endogenous components of cells and biomass have their own unique spectral features that can be identified. The hyperspectral image contains these signatures within pixels associated with distinct objects in the image. With the image viewer, individual pixels of objects can be selected and the spectra of those pixels saved to a database. The spectra are used with spectral classification methods in the software to determine the number and locations of similar objects in other samples. Hyperspectral imaging can also serve as a tool for “data mining” to determine optimal wavelengths and recording conditions for specific applications.
Sample applications
Applications for the HSI microscopy system include nanotoxicology, drug delivery, and biomass analytics. At the FDA’s National Center for Toxicological Research, Drs. Neera Gopee and Paul Howard realized the approach could provide benefits as a primary detection tool in research on dermal penetration of topically applied formulations including nanoparticles. They found HSI helpful for quantifying nanomaterials in tissue samples based on their unique spectral signatures (see Fig. 2) The approach provided a relatively simple and quick quantitative method for screening samples prior to using more time-consuming methods such as inductively coupled plasma mass spectrometry or electron microscopy. By collecting spectra of particles dispersed in liquid media, and then looking for these spectra in hyperspectral images of treated tissues, it is possible to quantify particle abundance and cluster size.
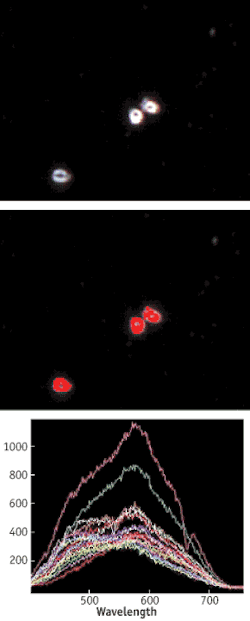
Similar methods have been demonstrated for using captured spectra to identify strains and possible sources of Anthrax spores (Fig. 3). Since there is a growing need to increase biofuels production from nonfood sources such as forest products, microbial activity during the fermentation process is being investigated with HSI methods. Cellulosic, hemicellulosic, and lignin components can be identified spectrally in thin slices of plant materials.
Additional uses now being investigated include targeted drug delivery with nanoparticles and quantum dots, and tumor-cell differentiation. In diagnostic imaging, HSI is being combined with colonoscopy, ocular funduscopy, and body scanning for detection of cancer and eye disease, and examination of skin ulcers. We expect HSI microscopy will begin to play a significant role in new contributions to basic and applied research, in a wide range of life-science disciplines.
James Beach is president of Willis Optics and associate professor at Louisiana State University Health Sciences Center (New Orleans, LA). He was the lead developer for the CytoViva hyperspectral microscope. Contact him at [email protected]; www.cytoviva.com.