Conventional images of biomolecules, cellular components, and similar targets come with two severe drawbacks: they exist in two dimensions and at a single point in time. Several research groups have now begun to develop optical technologies that yield images in extra dimensions. Three new methods in particular are providing new ways of viewing difficult-to-detect structures in three dimensions and monitoring them over time.
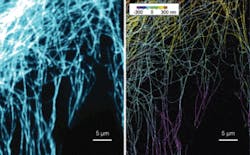
A group at Harvard University (Cambridge, MA) has adapted its stochastic optical reconstruction microscopy (STORM) fluorescence technique to provide extraordinarily clear 3-D images of cells’ interiors. Led by Xiaowei Zhuang, professor of chemistry and chemical biology and of physics and a Howard Hughes Medical Institute investigator, the team developed STORM in 2006. To apply it in its original form, scientists label the molecules they want to investigate with fluorescent probes. A burst of light activates the fluorescence and a microscope captures an image of the fluorescing probes. To ensure that the microscope images each fluorescing molecule separately, the technology excites only a small percentage of the molecules in each pass. Multiple repetitions provide images of each molecule under study. From that, scientists can calculate the location of every molecule in a cell with a 2-D resolution of 20 to 30 nm.
Unfortunately, a 2-D image can confuse as well as elucidate. Cellular components that appear close may lie at different depths. Fortunately, Zhuang explains, “images of individual molecules collected to generate a two-dimensional STORM image also contain information that reveals the molecules’ positions in the third dimension.” The team obtains that information by inserting a cylindrical lens into the microscope’s imaging path. “The image is round when the molecule is in the average focal plane and becomes elongated in the x or y direction when it is above or below the plane,” explains postdoctoral student Bo Huang. “The z coordinate of the molecule can be calculated by calibrating the relationship between the shape of the image and the z position.”
So far, the group has used the technology to image microtubules and clathrin-coated pits in 3-D (see figure). Next they plan to add the dimension of time to its imaging capability. That will involve speeding up the process, which currently takes from tens of seconds to minutes to create a single 3-D image. Faster technology will permit scientists to follow cells’ dynamic processes.
Harmonic holography
Holography offers another route to dynamic 3-D images of biological processes. For example, an international team has used second-harmonic signals generated by a femtosecond laser to produce holographic images rapidly enough to track fast-moving processes at the molecular level. The research team—consisting of Ye Pu, a postdoctoral scholar at the California Institute of Technology (Pasadena, CA), Martin Centurion of the Max Planck Institute of Quantum Physics (Garching, Germany), and Demetri Psaltis of Lausanne’s École Polytechnique Fédérale (Switzerland)—split 810 nm femtosecond laser pulses into a pump and a reference beam. Directed at appropriate nanocrystals, the pump produces a strong second-harmonic signal, which creates a holographic image of the crystals by interfering with the reference beam.
“This is the first time the principle of harmonic holography has been demonstrated,” Pu says.
The team plans to obtain holograms of molecular processes in cells. “We will attach certain molecules to nanocrystal tags,” Pu explains. “The tags will give a signal to let you know where the molecules are.” That will require some technical advances. “The crystals that produce second harmonics are now micro size. Our real goal is to obtain 10 nm crystal size,” Pu says. “We are also working on surface chemistry that will enable users of the technology to attach whatever molecules they want to the surface of the nanocrystals.” When that becomes possible, users will benefit from the technology’s speed to study dynamic processes. “In our holography technique, it takes only a single laser shot to generate a 3-D image.”
Another holographic method, Fresnel incoherent correlation holography (FINCH), applies holography to fluorescence. It uses microscopes with high resolving power, a spatial light modulator, a CCD camera, and simple filters to acquire 3-D microscopic images.
“It will be possible to obtain a 3-D image without scanning. And it will work with any wave-based imaging process in the electromagnetic spectrum,” says Gary Brooker, director of the Johns Hopkins University Microscopy Center.
So far, they have applied the method only to still images. “But with the FINCHSCOPE you can photograph multiple images at once, enabling you to capture a 3-D image of a moving object,” Brooker says.
About the Author
Peter Gwynne
Freelance writer
Peter Gwynne is a freelance writer based in Massachusetts; e-mail: [email protected].