Advances in fiber photometry help demystify the brain
A full, complete understanding of the brain’s complexities remains elusive. Decoding its mysteries holds the key to understanding neurological diseases—from Alzheimer’s and dementia to epilepsy and multiple sclerosis—and the ability to more effectively treat and, maybe someday, cure them.
Long relied-upon noninvasive imaging techniques such as MRIs and CT scans offer a look inside the brain, but methods using optical fiber are showing promise, especially fiber photometry.
Fiber photometry is an optical imaging technique for studying neural circuits, first developed in 2005 by a team at Ludwig Maximilian University of Munich (Germany) as a way to record cortical (the outer layer of the cerebrum) calcium ion (Ca2+) waves in resting newborn mice.1 It involves implanting optical fiber into the brain to observe and record neuron population-level Ca2+ activity from specific types of cells within different regions of the brain. In the brain, calcium activity is central in the control of activities like synaptic motion and memory formation.
Used in combination with genetically encoded calcium indicators, fiber photometry allows for real-time monitoring of neural activity.
“Fiber photometry provides a unique feature for an easy and stable recording of population activities with cell-type specificity in freely moving animals,” says Ruonan Fan, a Ph.D. candidate at Huazhong University of Science and Technology (Wuhan, China), where she works in the lab led by Ling Fu, assistant director of the Wuhan National Laboratory for Optoelectronics at Huazhong and a principal investigator with the Britton Chance Center for Biomedical Photonics.
Growing advantages
Although low temporal and spatial resolution represent a notable challenge, photometry remains the preferred method over other calcium imaging techniques such as one- or two-photon microscopy. Optical fiber is easier to implant and weighs less, too, allowing subjects the freedom of movement and natural behavior key to the technique. Fiber photometry is also more resistant to electrical interference, more efficient at collecting data, and has so far proven more stable for long-term neural monitoring.
“It has become a widely used optical method in neuroscience,” Fan says, citing the development of an extensible multichannel fiber photometry system by Fu’s group in 2015 that can simultaneously monitor neural activities in several brain areas of an animal or even in different animals.2
Another Fu lab-led study involved development of axonal terminal-specific multichannel fiber photometry in 2019.3 Axons transmit information from one neuron to another mainly through synapses at their terminals, the group explains in the study, and “recording from axonal terminals in freely moving animals is an essential step to understand information processing during animal behaviors.”
Now, ongoing research (first published in May this year) led by Fu’s group is providing even more advantages, building on the conventional technique.4
“We developed an all-fiber-transmission photometry system that is compatible and flexible,” says Fan. “And we could achieve simultaneous optogenetic manipulation and multicolor recording of neuronal activities and the neurotransmitter release in freely moving animals.”
The team has been looking at manipulating and real-time monitoring neuronal activities with cell-type specificity and precise spatiotemporal resolution during animal behavior. “These are fundamental technologies for exploring the functional connectivity, information transmission, and physiological functions of neural circuits in vivo,” Fan says.
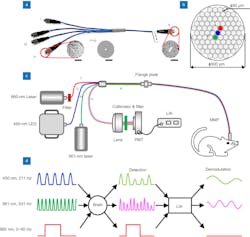
The new all-fiber-transmission photometry system is based on a multibranch optical fiber bundle (see Fig. 1). It allows simultaneous optogenetic manipulation and multicolor recording of neuronal Ca2+ (or neurotransmitter signals) in freely moving animals.
“This is an effective supplement to current fiber photometry systems,” Fan says.
The new system has achieved full coverage of the visible spectrum for polychromatic excitation and optogenetic manipulation, as well as all-fiber transmission of excited and emitted light. Fan says this was done specifically by combining a non-wavelength selective four-branch fiber bundle that can realize multicolor recording while also supporting precise optogenetic stimulation (see Fig. 2). The bundle is combined with a photomultiplier tube (PMT) to replace the traditional imaging structure. A custom-designed lock-in amplifier is used, as well, to accurately separate two fluorescence signals with different wavelengths detected by the PMT and effectively suppress the optogenetic stimulation-induced artifacts and channel crosstalk.“We performed a series of experiments, which indicated the system had excellent light transmission performance. It was capable of effectively exciting and collecting fluorescence signals, and the collection efficiency was 20% to 30% better than a traditional system,” Fan says.
The team found no substantial channel crosstalk, and successfully recorded both neuronal Ca2+ and neurotransmitter dynamic signals in the nucleus accumbens (NAc)—a structure comprised mainly of medium spiny neurons that are distinguished by their relative expression of dopamine receptors—while at the same time applying precise optogenetic manipulation of the dopaminergic terminals in the same site in a freely moving mouse. The researchers were also able to inhibit the stimulation artifacts—short-duration, high-amplitude neural spikes that cause noise interference because they are non-physiological—to a basal noise level while recording (see Fig. 3).“With its advancement, fiber photometry is increasingly popular among neuroscientists as a convenient tool for the recording of genetically defined neuronal populations,” Fan says. “And subsequent improvements can mostly focus on different application demands.”
Earlier fiber photometry techniques and systems to optogenetically manipulate or record neuronal activity are usually separated and work independently, and they are only capable of manipulation and single-channel recording at a single wavelength. Another challenge is that stimulation artifacts occur during multicolor recording and manipulation due to the overlap between the excitation spectrum of photosensitive proteins and the emission spectrum of the genetically encoded calcium indicators.
“To achieve simultaneous optogenetic manipulation and dual-color recording, more wavelength-range fluorescent proteins with spectral spacing need to be involved,” Fan says. “The optical system is thus required to fully cover the visible spectrum.”
But Fan adds this is a problem with currently available fiber photometry systems because they only cover the spectrum of 405 to around 600 nm by using the classical epifluorescence imaging architecture, which consists of an objective lens and two or three dichroic mirrors. “In this case,” she says, “more dichroic mirrors are needed to couple the multiple wavelength light beams into one optical fiber, which makes the system more complex to extend the spectrum.”
The new all-fiber-transmission photometry system can be used to “further observe the activity of different types of neurons and simultaneous optogenetic manipulation and multicolor recording to monitor the feedback effect caused by the intervention of optogenetic in neural circuits,” Fan says. “This is now recognized as ‘the method of dreams’ for the causal investigations of neural circuits and neurological diseases.”
REFERENCES
1. H. Adelsberger, O. Garaschuk, and A. Konnerth, Nat. Neurosci., 8, 8, 988–990 (Aug. 2005); doi:10.1038/nn1502.
2. Q. Guo et al., Biomed. Opt. Express, 6, 10, 3919–3931 (Sep. 10, 2015); doi:10.1364/boe.6.003919.
3. H. Qin, J. Lu, W. Jin, X. Chen, and L. Fu, Neurophotonics, 6, 3, 035011 (Jul. 2019); doi:10.1117/1.nph.6.3.035011.
4. Z. Qi et al., Opto-Electron. Adv., 210081 (May 2022); doi:10.29026/oea.2022.210081.
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.