AI-assisted imaging noninvasively discerns tumors
Colorectal cancer—one of the most common forms of the disease—is not often detected early enough via conventional methods. But researchers at the Champalimaud Foundation in Portugal are working to change this with an imaging approach that provides real-time, noninvasive, comprehensive tissue characterization (see video).
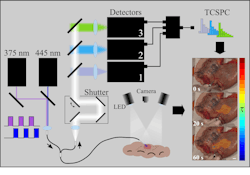
The approach uses a fiber-optic probe that excites tissue samples at two specific wavelengths—375 nm and 445 nm—in a multiplexed fashion. This dual excitation captures time-resolved autofluorescence signals from endogenous molecules such as NAD(P)H (essentially an electron donor in all organisms), flavins, and collagen.
“By analyzing the fluorescence decay times, our method provides insights into the tissue’s biochemical composition without the need for exogenous contrast agents,” says João Lagarto, head of Biophotonics at the Champalimaud Foundation.
How does it all work?
The team’s setup features two picosecond-pulsed laser diodes offering periodic excitation at the 375- and 445-nm wavelengths. Each group of pulses corresponds to a single fluorescence lifetime measurement, which the team says produces an acquisition rate of 50 Hz. Excitation light can then be delivered to the specimen via a single 300-µm excitation multimode fiber at the center of a custom optical fiber bundle.
“Fluorescence lifetime data are analyzed using the phasor method,” Lagarto says, noting that this “transforms complex decay curves into a simplified graphical representation, facilitating the identification of distinct tissue components.”
The phasor plot enables the differentiation of normal, adenomatous, and malignant tissues based on their unique autofluorescence lifetime signatures. To classify the tissue types, the team’s work also relies on a supervised ensemble learning model that uses histopathology diagnosis.
“Our approach enhances the system’s ability to distinguish between benign and malignant tissues based on the phasor-derived features,” Lagarto says.
More ‘pros,’ fewer ‘cons’
The team’s integrated imaging and analytical approach is label-free, meaning no chemical dyes or contrast agents are needed, which in turn reduces potential patient risks. It also offers real-time analysis, expediting assessment during procedures and aiding precise lesion delineation.
Enhanced diagnostic accuracy is another advantage of the new system, as is the ability to comprehensively characterize tissue by capturing biochemical and structural features that can be further used for evaluating molecular characteristics of the tumor and for outcome prediction.
Lagarto explains there are also several key challenges that exist with current colorectal cancer detection and monitoring methods, which his team’s approach overcomes.
Traditional autofluorescence intensity imaging often suffers from low contrast and is affected by tissue scattering, making it difficult to reliably distinguish between benign and malignant tissues. “By using fluorescence lifetime imaging and phasor-based analysis, our method provides robust, intensity-independent differentiation of tissue types,” Lagarto says.
Another challenge: Standard methods often provide limited functional or molecular information, focusing primarily on morphology. In contrast, the team’s approach delivers comprehensive tissue characterization and captures biochemical and structural features that can inform molecular profiling and outcome prediction, which supports personalized monitoring and treatment planning.
“Beyond surgical oncology, this approach is intended for use during index colonoscopy for early detection,” Lagarto says. “It’s designed for treatment follow-up, as well, which provides a noninvasive tool to monitor disease progression, assess therapy response, and support personalized management strategies.”
What’s on tap?
The team is now working to further validate their findings, via clinical trials in index colonoscopy and post-treatment monitoring, refinement of imaging and analysis, integration with molecular and genomic data, outcome prediction, and risk stratification. They’re also taking steps to develop predictive models that correlate imaging-derived tissue features with clinical outcomes, recurrence risk, and therapy response, to support tailored follow-up and treatment strategies.
“This work has significant implications for the future of cancer detection and management,” Lagarto says. “By providing real-time, noninvasive, comprehensive tissue characterization, it enables precise monitoring of therapy response, which allows clinicians to evaluate how a tumor reacts to treatment without repeated biopsies.”
The detailed biochemical and structural information the team captures also informs personalized medicine, which can guide treatment choices tailored to the specific molecular and functional profile of each patient’s tumor. It can also correlate tissue features with clinical outcomes, and offers a powerful tool for outcome prediction to help identify patients at higher risk of recurrence or progression.
“We hope that this approach will support a shift toward more dynamic, individualized, and predictive cancer care, improving both treatment effectiveness and patient management,” says Lagarto.
FURTHER READING
J. L. Lagarto et al., Biophotonics Discov., 2, 3, 032705 (Jul. 2025); https://doi.org/10.1117/1.bios.2.3.032705.
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.