A multidisciplinary team led by Jerry Chen, a neuroscientist and assistant professor of biology at Boston University (BU; Boston, MA), has developed a new approach to tracking neural activity in living animals. It involves, in part, voltage imaging (see video). A technique gaining traction within the neuroscience community, it enables direct fluorescence imaging of changes in voltage and electrical activity—how neurons communicate with each other and process information—in the brain.
“Using light to image this electrical activity has been a dream of neuroscientists for decades,” Chen says. “Voltage imaging has been an ongoing area of research, but only recently have things really taken off.”
The technique involves expressing genetically encoded indicators that can change fluorescence in response to neuronal activity, and essentially operates at the fundamental limits of biology and physics. It’s a promising method that allows researchers to better visualize and understand neuron firing, cognition, and behavior.
But it’s not without hurdles.
Conventional challenges
A conventional way to measure activity deep in the brain involves electrodes. They’re implanted into the brain, which is a highly invasive process, and produce electrical impulses delivered to specific areas.
Calcium imaging is another method to measure brain activity. It’s a microscopy technique to look at calcium indicators, which are fluorescent molecules that respond to the binding of calcium ions by fluorescence properties. This method is typically used to track neurons’ activity over a period of time, allowing researchers to investigate how these networks change and grow.
Voltage imaging can complement traditional electrode-based methods or calcium imaging, although it requires kilohertz sampling rates that reduce fluorescence detection, resulting in noisy images—known as shot noise. The amount of light involved in shot noise is very low, and it’s a longstanding fundamental limitation in microscopy that hinders reliable measurements using conventional technology. High-photon flux excitation can overcome photon-limited shot noise, Chen and fellow researchers note, but photobleaching and photodamage restrict the number and duration of simultaneously imaged neurons.
And although voltage imaging is less invasive and has demonstrated more accuracy, it too comes with risk factors and challenges.
Voltage imaging requires shining a lot of light into the brain, which can lead to overheating and photodamage. This method also has a limited capacity—it’s only able to image 10 neurons at a time. Any more than that, and the technique’s efficiency suffers.
Another major challenge is being able to maximize the signals received and the amount of neurons recorded, while also minimizing photodamage and preventing damage to the brain.
Working in conjunction with a multidisciplinary team of researchers, Chen and fellow BU optical engineers are offering an approach that could be the answer to walking that line.
A winning combination
In their work, the team investigated an approach involving two-photon flux, brighter light conditions. They’ve been able to denoise images that, because of low-light conditions, can be shot noise-limited. This is thanks to efforts by Lei Tian, an assistant professor in BU’s Department of Electrical and Computer Engineering, who developed a denoising algorithm called DeepVID that infers fluorescence from shot-noise-limited signals. Breaking the shot noise limit is analogous to breaking the sound barrier in aeronautics, Chen notes.
“The denoising approach Prof. Tian developed can break that limit,” he says, calling the new algorithm a game-changer. “And it allows us to recover more information from our images than previously thought.”
Conventional imaging approaches can either image a small number of neurons very quickly or many neurons very slowly, according to Chen. Combining the denoising algorithm with a microscope he developed with his lab of fellow optical engineers—with guidance from others at BU’s Neurophotonics Center and Micro Nano Imaging Facility—allows the team to do both: image large numbers of neurons very quickly.
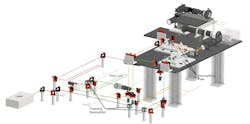
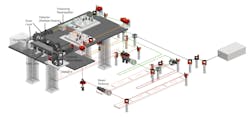
The microscope, which the team calls SMURF, can image 100 neurons at a time at 1000 Hz (see Figs. 1 and 2). “This is fast enough to image electrical activity,” Chen says, noting that they need to get just enough light into the brain to get fluorescence signals out.
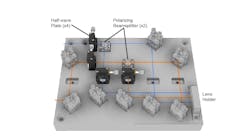
It’s a two-photon microscope that enables kilohertz frame rate imaging across a 0.4 × 0.4 mm field of view. The technology changes fluorescence in response to neuronal activity and allows imaging at 1000 frames per second (see Fig. 3).
Genetically encoded voltage sensors featuring improved spike detection are another piece of the combined framework. They were developed in the lab of Vincent Pieribone, a professor of neuroscience and cellular and molecular physiology at Yale University (New Haven, CT). His lab also developed new proteins expressed in the neurons that allow the researchers to image electrical activity in the brain more clearly and easily.
Furthering the research
With the unique approach comprising the new microscope, denoising algorithm, and voltage indicators, the researchers say they have achieved “simultaneous high-speed, deep-tissue imaging of more than 100 densely labeled neurons over one hour in awake, behaving mice.” Chen says this demonstrates a scalable approach for voltage imaging across a larger number of neuronal groups.
Further expanding on the components of the combined technique is expected to further boost the number of neurons the team can image—from a standard 10 neurons to the 100 they’ve already demonstrated is possible, Chen says, to potentially thousands. This will ultimately provide neuroscientists with a better understanding of how the brain processes information.
“We can apply this technology to answer basic questions about how the brain works,” Chen says. “It really opens up a new world of possibilities for imaging more neurons in the brain at higher speeds than previously thought possible.”
The combined approach, which was intended to make voltage imaging more efficient, practical, and scalable for research, may enable more comprehensive studies of neurological diseases involving disruptions in electrical and neuronal activity.
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.