Piezophototronics quantify subcellular force distribution
According to CDC statistics, in 2019 more than 600,000 Americans died of some form of heart disease. Many of those deaths can be traced to failure of normal function in cardiomyocytes—the roughly 3 billion specialized muscle cells that make up about 70% of the human heart. To understand the mechanism underlying many cardiovascular diseases and identify potential therapeutic targets, it’s necessary to have insight into the structure and function of cardiomyocytes.
Cardiomyocytes are smooth muscle cells with bundles of actin and myosin fibers that form contractile sarcomeres and are assembled into striated myofibril structures. Myofibrils contract and relax in response to calcium ion levels, which are in turn regulated by depolarization of the sarcolemma—the lipid cell membrane. Membrane tubules extend into the myocyte body to allow more uniform calcium influx, helping synchronize myofibril contraction and relaxation. The structural complexity means that true understanding of cardiomyocyte function requires quantification of force on a subcellular level.
That’s exactly the function performed by a novel piezophototronic light nanoantenna array developed by Professors Zhou Li, Junyi Zhai, and Zhong Lin Wang of the Beijing Institute of Nanoenergy and Nanosystems at the Chinese Academy of Sciences and their colleagues at several other institutions.1
Getting bent out of shape
Piezophototronic engineering links semiconductor physics, photoelectric phenomena, and piezoelectric effects. In these devices, mechanical stress induces electronic charge redistribution, the new charge distribution modifies semiconductor bandgap configuration, and that in turn influences the material’s interaction with light. This particular incarnation is built on nanopillars, 150 nm in diameter and 1.5 µm tall. Near the top of the nanopillar, an indium gallium nitride/gallium nitride multiple quantum well (MQW) region is fabricated, which, when excited by 405 nm light, photoluminesces at 460 nm.
When the MQW is subject to mechanical stress—either tension or compression—charge centers within the crystals are displaced, resulting in a net polarization that displaces charge carriers. When a pillar is bent, for example, one side will be in tension and the other in compression, adding either negative or positive charges at the MQW interfaces, reducing the photoluminescence efficiency in either case.
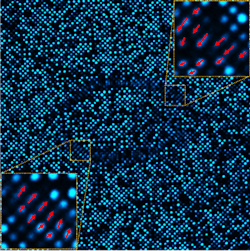
The researchers fabricated an array of these nanopillars, which they called piezophototronic light nanoantennas (PLNAs). The PLNAs are separated and spaced 800 nm center-to-center, resulting in a PLNA density of 31,750 dpi. The photoluminescence intensity of any given PLNA decreases as a function of its “bending.” With the array illuminated by a laser diode emitting at 405 nm, confocal microscopy at 460 nm provides instantaneous measurement of that bending. The array showed high photostability, demonstrating minimal change in photoluminescence over 100 hours of imaging in cell culture conditions.
With the array built and validated, the next step was to adhere a cardiomyocyte to the PLNAs. Seeding the array at 10,000 cells/cm2 allows single cells to attach to the PLNA array. Integrin dimers adhere to individual PLNAs and cross the cell membrane, where they link through a few intermediary molecules to actin fibers in the cytoskeleton. As the cell contracts, the actin fibers exert transverse force on the PLNAs, bending them and reducing their photoluminescence efficiency, which can be instantaneously measured (see figure).
“It’s still a huge challenge to obtain a real-time, dynamic, and high-resolution mapping of the force distribution across a single living cell,” Li says.
The PLNA array is a mechanical-optical coupling methodology for real-time force imaging with submicron spatial resolution and high sensitivity, with no need for electrical interfaces.
A tool for drug development
To establish the method and verify the feasibility, the research team acquired real-time images of the cell traction force (CTF) of single cardiomyocytes. As a cell spontaneously contracts and relaxes, the cell-bound PLNAs beneath it change their intensity (along with a slight shift in position) on the nanosecond timescale, so the method’s acquisition speed is essentially limited only by image capture. Using a combination of deterministic modelling and device calibrations, the researchers linked photoluminescence measurements directly to force, providing quantitative CTF measurements in real time at a 0.8-µm resolution.
“Previous CTF measurement methods are either restricted for probing several scattered points on a cell surface or limited in spatial resolution and temporal resolution, which are dramatically important for kinetic cells such as cardiomyocytes,” Li says.
Because cell condition is affected by disease, drugs, external physical stimulations, and other processes, Li believes real-time CTF quantification can be used as a tool for research and potential clinical utility. The researchers will next examine the mechanical effects of drugs on cardiomyocytes.
According to these research groups, each process in the development was critical: fabrication of the PLNA, primary culture of a single cardiomyocyte, and construction of the mechanical model. But it is rewarding as well.
“Our work,” Li says, “has effectively filled the shortcomings in this field.”
REFERENCE
1. Zheng et al., Sci. Adv., 7, 22, eabe7738 (2021); doi:10.1126/sciadv.abe7738.
About the Author
Richard Gaughan
Contributing Writer, BioOptics World
Richard Gaughan is the Owner of Mountain Optical Systems and a contributing writer for BioOptics World.