Optical Coherence Tomography: OCT angiography’s gains
OCT angiography (OCTA) "stands to change the practice of ophthalmology in the next 10 years as profoundly as OCT has changed it in the past 10 years," according to a comprehensive review of current methods and clinical applications.1 That is quite a statement, given the incredible advances OCT has brought to ophthalmology.
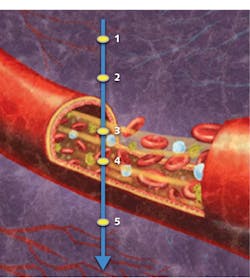
What accounts for this striking forecast? Whereas traditional optical coherence tomography (OCT) is able to depict structures quickly and noninvasively in three dimensions, OCTA images retinal vasculature at near-histology level resolution, and delivers depth-resolved information never before available1—also quickly, noninvasively, and in 3D. It is a functional imaging technique that depicts blood flow by measuring variation between two OCT signals—one backscattered from unmoving structural tissue and the other backscattered from red blood cells traveling through vessels (see Fig. 1).
As interest in OCTA has grown, so has the development of algorithms that leverage different OCT signal components to highlight the operation of micro-vasculatures. These algorithms have found application in dermatology, oncology, and neuroscience, as well as ophthalmology2—the application for which OCTA received FDA clearance in 2015.
Since then, OCTA has made steady progress, not only in terms of algorithm development and applications research, but also in terms of commercial ophthalmic instrumentation, and clinical adoption and proficiency for retinal scanning.
Instrumentation development
About a year before Carl Zeiss Meditec (Dublin, CA) received the first FDA 510(k) clearance of OCTA technology for its AngioPlex system, OptoVue (Fremont, CA) launched its AngioVue Imaging System outside the U.S. Then, in early 2016, OptoVue announced FDA 510(k) clearance of AngioVue, which by that time was in use at more than 525 non-U.S. clinical sites. Wasatch Photonics (Logan, UT) released WP MicroAngio, an imaging device targeting research and original equipment manufacturer (OEM) markets, and Topcon (Tokyo, Japan) introduced the SS OCT Angio add-on for non-U.S. customers of the company’s swept-source DRI OCT Triton system.
Later in 2016, Zeiss announced the first FDA clearance for swept-source OCT imaging technology for posterior ocular structures: Zeiss Plex Elite 9000 was launched as an OCT/OCTA platform for advanced retina research (see Fig. 2). And Heidelberg Engineering (Heidelberg, Germany) announced the OCT Angiography Module, designed to add OCTA capability to upgradeable versions of its Spectralis system, for sale outside of the U.S.In early 2017, Zeiss introduced an update to Plex Elite 9000, developed in collaboration with the Advanced Retina Imaging (ARI) Network, a collaborative Zeiss established to facilitate the exchange of ideas and findings among Plex users and to advance OCT technology innovations and clinical applications. The 200 kHz ultra-wide HD Plex system offers a field of view up to 70°, and new software aims to advance assessment of diabetic retinopathy (DR) and retinal vein occlusion (RVO). To entice prospective customers, Zeiss has offered free software and hardware upgrades for the next several years, including an imminent upgrade expected to double laser speed to 200,000 A-scans (z-axis scans) per second, with faster scanning rates anticipated.
Also in 2017, Optovue debuted AngioVue Essential, a system targeting optometry and general ophthalmology practices that generates reports showing individual layers of retinal vasculature alongside structural OCT B-scans (optical cross-sections), which clinicians can use as a patient-communication tool to take basic eye exams to a new level (see Fig. 3). And Heidelberg announced upgrades to its OCTA module: improved image quality for visualization in the deep vascular complex, refined retinal layer segmentation, and a tool for removing projection artifacts (cast by superficial vasculature). Other OCT instrumentation developers, such as Optopol and Nidek, have demonstrated OCTA platforms, so it seems likely more instruments will come online in the near future.Thanks in large part to commercial development, OCTA has gained momentum in terms of adoption and clinical experience. Considering such gating factors standard for new technologies as lack of clarity regarding when to use OCTA vs. the traditional approaches, and lack of approval for insurance coverage of OCTA specifically, eye-care professionals have been quick to embrace OCTA.1,3 This is evident by the fact that ophthalmic meetings now frequently feature imaging sections dedicated to OCTA, and new meetings have even been established to address clinical and translational research in OCTA.3
Versus traditional angiography
The traditional approach to retinal angiography—which facilitates the monitoring of conditions and disease diagnosis by allowing visualization of critical structures at the back of the eye—involves the intravenous administration of dyes. Fluorescein angiography (FA) enables visualization of a patient’s retina (the light-sensitive tissue layer at the back of the eye), while indocyanine green (ICG) highlights choroid vasculature (blood vessels beneath the retina). Associated risks are rare, but they can be serious, and in any case, the procedure is invasive, relatively time-consuming, and provides only snapshot imagery.4 By contrast, OCTA is noninvasive, carries no risk at all,2 and reveals dynamic processes. In fact, the emergence of OCT in combination with drawbacks of the conventional approaches has seen a dramatic decline in the use of FA.3
For monitoring retinal disease complications such as DR and RVO that involve the macula—the portion of the retina that governs the center of our visual field and allows us to see straight ahead—OCTA is at least as good as traditional approaches.2 It’s true that OCTA cannot detect leakage in the retina, as do the invasive dye approaches, but the new technique may yet provide further insight into the pathophysiology of retinal disease, and a means of routinely monitoring disease progression to guide treatment.2
Enabling tech drives progress
A number of optics- and photonics-based developments will enable OCTA to better address clinical needs.
Current OCTA systems are based on either spectral domain (SD) or swept source (SS) OCT, and the latter has proven better able to detect choroidal neovascularization (CNV), a hallmark of such diseases as age-related macular degeneration (AMD). However, SS-based systems have a ways to go in terms of clinical development.1
Field-of-view (FOV) expansion is important because research is showing that in diabetic retinopathy (DA), for instance, early changes in blood flow and vessel morphology take place in the periphery of the retina, which may be outside the scanning area of current clinical OCTA systems.2 This is an issue that Zeiss addressed with the 12 mm2 FOV Plex Elite 9000 and that montaging algorithms such as OptoVue’s AngioVueHD Montage also look to tackle. While Plex Elite is a research instrument designed to help scientists understand OCTA’s clinical potential, FOV will likely continue to advance in commercial systems.1
A related need is greater speed. The viability of clinical OCTA depends on the time a patient is able to remain still without blinking—typically 3–6 seconds.2 Faster image-acquisition speed will facilitate scanning over a wider area while retaining the ability to detect slower flow.2
In addition, OCTA could benefit from systems that implement longer-wavelength light, optimized by application.2 Light centered on 1050 nm, for instance, rather than the 800 nm typical for ophthalmology applications, will allow OCTA to achieve greater penetration into ocular tissue, and thus provide better flow information for deeper structures.2 Similarly, in dermatology applications, a wavelength range centering on 1680 nm (rather than the standard 1300 nm) would allow deeper penetration into the dermis.2
Motion correction is important for revealing true flow information, and a method able to remove tissue-bulk motion that produces false positives, and to generate motion-free data without increasing scan time or adding artifacts, will facilitate both clinical and preclinical application of OCTA.2 In addition, the ability to reveal flow data hidden by the projected artifacts will provide more complete information.2
Finally, signal quantification is perhaps OCTA’s holy grail. And while researchers are working on this, it seems that a satisfying solution has yet to be found—so further work is needed here as well.
The eyes—and more—have it
OCTA has expanded the potential applications of OCT, and enabled insight into blood-flow changes related to progression of disease.2
For retinal diseases like AMD and others that clinicians have traditionally addressed using invasive dye studies, it seems likely that OCTA will increasingly become the standard for diagnosis and management.1
But OCTA also provides a novel means of assessing the peripapillary capillary plexus, a structure important in glaucoma that no other commercial imaging modality is able to highlight.1
While much work remains to facilitate clinical adoption, enthusiasm for OCTA is strong.3 And with its promise for application in dermatology, neurology, and oncology applications as well, it’s safe to say that OCTA has a promising future and that its capabilities imply great promise for patients.
REFERENCES
1. A. H. Kashani et al., Prog. Retin. Eye Res., 60, 66–100 (2017).
2. C. L. Chen and R. K. Wang, Biomed. Opt. Express, 8, 2, 1056–1082 (2017).
3. J. P. Ehlers, Ophthalmol. Retina, 1, 6, 457–460 (2017).
4. C. L. Chen, Q. Zhang, A. Zhang, and R. K. Wang, "OCT angiography: A new approach with ‘gold standard’ capabilities and more," Laser Focus World, 51, 11, 58–61 (Nov. 2015).
About the Author

Barbara Gefvert
Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.