Novel Detectors: Ultrathin light-trapping birnessite could be sustainable energy source
Splitting water (H2O) through an oxidation process into hydrogen fuel and oxygen or converting carbon dioxide (CO2) into a fuel such as methanol requires a large amount of energy. Fortunately, in nature, plants perform photosynthesis by using light to perform a set of chemical/electrical processes that convert energy to food and oxygen.
But a new photosynthesis method from researchers at Florida State University (FSU; Tallahassee, FL) explores the substitution of various cations into a layered manganese oxide (MnO) material called birnessite to tune the bandgap of the resultant modified material such that it is able to absorb light and efficiently complete a water-splitting process that can generate hydrogen.1 The research has implications for low-cost methods to create hydrogen gas (H2) and oxygen gas (O2) using engineered ultrathin materials.
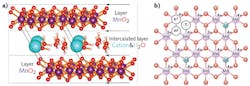
Birnessite intercalation
The process of substituting cations into interlayer centers within the molecular structure of a material is called intercalation. Birnessite, a natural material known to act as a catalyst in a water-splitting process, is composed of layers of MnO in an octahedral form with an interlayer of hydrated cations and has the general formula Na0.5MnO2·1.5H2O (see figure).
To understand whether birnessite can act as a promising light-capture material for photosynthesis, the researchers studied the resultant bandgap and electronic structure of birnessite by substituting various cations (including lithium [Li+], potassium [K+], and aluminum [Al3+], among others) between the birnessite layers, on single-layer ultrathin surfaces, with and without water molecules, and by varying the arrangement of the MnO layers.
Analysis of the van der Waals forces between molecules and water-depleted molecules substituted with various cations revealed that ultrathin structures with strontium, calcium, boron, or aluminum have valence bands with a band alignment suitable for water splitting. The role of the various cations is to keep charge neutrality. However, the layers interact through van der Waals forces with interaction values of only 23 kcal/mol, meaning they are not held strongly to each other and layers can be peeled off in the same way graphite is peeled to obtain graphene.
In one arrangement, intercalated boron in an anhydrous environment has semiconducting behavior with a direct band transition for light capture of 2.01 eV, indicating that the material with this cation can capture sunlight efficiently and this energy can be used to drive the transformation of water to O2 and H2. And because the elements that make up this compound are abundant, the process can essentially be cost-effective, commercially feasible, and scalable.
Furthermore, for surface structures, magnesium and strontium have a direct transition of 2.19 and 2.69 eV, respectively, indicating high potential for strong light absorption and water splitting.
"This family of materials can do two things: absorb sunlight effectively and drive the conversion of water to oxygen and hydrogen while also serving as the catalyst for this conversion," says Jose L. Mendoza-Cortes, FSU assistant professor. "Essentially, this process does what any leaf in the planet can do—take sunlight and convert it into fuel that can be stored and used during periods when sunlight is not present."
REFERENCE
1. K. P. Lucht and J. L. Mendoza-Cortes, J. Phys. Chem. C, 119, 40, 22838–22846 (2015).
About the Author

Gail Overton
Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.