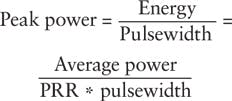Ultrafast lasers: Ultrahigh peak power femtosecond lasers advance bioimaging
JULIEN KLEIN
Traditionally, confocal and multiphoton-excited fluorescence (MPEF) microscopy images are formed by scanning a near-diffraction-limited, micron-size laser spot over a volume of interest that measures hundreds of microns. The latest multiphoton microscopes are equipped with fast galvanometer or resonant scanners able to generate hundreds of frames per second. The information in each voxel is acquired by accumulating the fluorescence resulting from the nonlinear absorption of many femtosecond (fs) laser pulses in the near-infrared (NIR) wavelength range, typically 900-1300 nm. To ensure that a sufficient number of consecutive laser pulses contribute to each voxel, the ultrafast source should have a relatively high (greater than 20 MHz) pulse repetition rate (PRR).
Conversely, the practical driver for fluorescence intensity, image brightness, and contrast in MPEF microscopy is the laser peak power delivered to the sample. Laser peak power is a measure of instantaneous photon density:
For most bioimaging applications-especially in vivo imaging tasks-researchers limit laser average power to hundreds of milliwatts (mW) at most to prevent specimen damage through heating and/or phototoxicity. Peak power (and consequently the fluorescence intensity) can be increased by decreasing either the PRR or the pulsewidth. For practical purposes, nearly all laser scanning multiphoton microscopes today are equipped with ultrafast lasers operating at a PRR between 50 and 100 MHz and peak powers on the order of hundreds of kilowatts-the sweet spot for today's point-scanning MPEF imaging applications.
Enabling possibilities
Leading research groups are now pushing the application envelope beyond these established paradigms. Commercially available ytterbium-based, high-peak-power ultrafast laser sources are enabling a new wave of multiphoton imaging modalities. These new laser platforms offer comparatively lower PRR (10 kHz-10 MHz) and pulse energies from hundreds of nanojoules (nJ) to tens of microjoules (μJ), resulting in impressive peak powers of 1-100 megawatts (MW). Such high peak power levels allow researchers to tailor and/or expand the excitation field, or distribute peak power over multiple imaging spots.
It is important to emphasize that gains in peak power are achieved by scaling up pulse energy, not average power. Indeed, these lasers offer a sustainable path to ultra-high peak power while operating at moderate average power levels (1-5 W) that are compatible with the imaging of fragile structures-structures particularly susceptible to heating, phototoxicity, and photodamage associated with excessive average power.
Ytterbium laser options
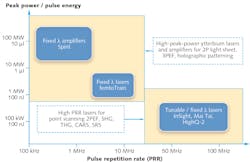
Ytterbium-doped lasers and amplifiers offer a unique blend of simplicity, compactness, and cost efficiency that differentiate them from the traditional widely tunable ultrafast lasers based on Ti:sapphire and optical parametric oscillators (OPOs). Ytterbium-doped gain media (bulk crystal, thin disk, or fiber) are energized directly with high-brightness infrared pump diodes. Mode-locking of ytterbium-based lasers produces ultrafast pulses in the 1020–1070 nm spectral range, with pulsewidths in the hundreds of femtoseconds. Such oscillators can be built with a high PRR compatible with point scanning microscopy (e.g., 63 MHz for Spectra-Physics' HighQ-2 laser),1 or with a longer optical cavity and lower PRR to generate higher pulse energy and higher peak power (for instance, 10 MHz, 300 nJ, and 1 MW, in the case of the Spectra-Physics femtoTrain). Ytterbium-doped regenerative amplifiers are also readily available to reach even higher pulse energy and peak power levels (for example, single shot -1 MHz, up to 40 μJ, and 100 MW, as with the Spectra-Physics Spirit platform). Figure 1 illustrates the tradeoff between high PRR and high peak power from a laser technology and application standpoint.
An important limitation of ytterbium ultrafast lasers and amplifiers is the lack of spectral tunability, which limits their use to applications compatible with 1 μm excitation. To alleviate this limitation, an optical parametric amplifier (either collinear OPA or noncollinear OPA) may be pumped with the second harmonic (~520 nm) or third harmonic (~347 nm) of the ytterbium amplifier 1 μm emission wavelength. This allows provision of complete spectral coverage from the ultraviolet to the mid-infrared in a compact, two-box solution that enables applications requiring both high peak power and spectral flexibility.
Peak power levels of a megawatt or more that are available from ytterbium lasers are suitable for a host of new peak power "hungry" bioimaging applications, such as:
- Two-photon selective plane illumination microscopy (SPIM), also known as two-photon light sheet microscopy;
- Multi-beam two-photon excitation and imaging, as utilized, for example, in a two-photon spinning disk laser scanning microscope;2
- Three-photon excited fluorescence microscopy (3PEF); and
- In optogenetics, holographic patterning, and temporal focusing for simultaneous photoactivation of large populations of neurons.
Let's take a closer look at some of these applications.
Bright light-sheet future
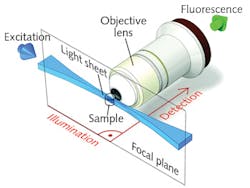
In two-photon SPIM, a two-dimensional thin sheet of light is created by focusing an ultrafast laser beam through a cylindrical lens, or by rapidly scanning a focused beam in one transverse direction (virtual light sheet). The generated fluorescence is collected in the direction orthogonal to the sheet through an objective lens, and is typically detected by a CCD camera (see Fig. 2). The sheet can then be scanned in the orthogonal dimension to illuminate the entire three-dimensional volume.3,4Compared to point-scanning MPEF, SPIM offers high acquisition speed, which is especially important for large samples and for controlling photodamage. Moreover, a two-photon light sheet offers the usual benefits of nonlinear excitation; i.e., enhanced penetration in live tissue (thanks to limited scattering) and enhanced longitudinal resolution (with help from better excitation confinement). High-peak-power lasers with PRR of 1 MHz allow for the creation of a light sheet with distributed peak power sufficient to generate a strong fluorescence signal across the entire sheet. Scanning the light sheet across the sample volume at relatively low speed eliminates the need for voxel averaging at high PRR, and is compatible with "slow" (megahertz and below) repetition rates. For these reasons, ytterbium-doped femtosecond amplifiers (with or without OPA for wavelength tunability) may be suitable for upcoming two-photon SPIM applications.
Deeper imaging with 3PEF
In recent years, three-photon excitation fluorescence (3PEF) microscopy has been proposed as a promising imaging modality to increase penetration depth in vivo, especially in neuroscience for the functional imaging of subcortical structures.5 The three-photon absorption process dictates that the laser light be shifted further into the NIR (typically to 1.3 μm and in some cases up to 1.7 μm), enabling greater reduction of scattering effects and allowing ballistic photons to make their way through the live tissue to the imaging plane.
The nonlinear three-photon process is a very low-probability quantum event that requires the simultaneous presence and absorption of three NIR photons to generate a fluorescence signal. Researchers rely on high-peak-power ultrafast lasers to compensate for such low probability. Ytterbium-doped ultrafast amplifiers-equipped with an OPA or NOPA to shift the excitation to the adequate wavelength-offer a robust and dependable solution.
Optogenetics with holography
As a final illustration of leveraging the high peak power of ytterbium lasers to push the boundaries of biological imaging, let us turn again to neuroscience-this time in the context of optogenetics.
In optogenetics, researchers utilize light-sensitive proteins (opsins) to selectively control the activity of neurons. Animals can be genetically modified so that specifically targeted categories of neurons express these opsins in the membrane, making them "activatable" when illuminated with light with the appropriate spectral characteristics. Certain opsins allow for the firing of an action potential and the generation of transient electrical signals, which are the basis for neuronal communication, while others can inhibit such firing. The most popular and commonly used opsins (channelrhodopsin-1, ChR2) are optimally excited in the two-photon regime at 920-950 nm. More recently, new red-shifted opsins enable excitation at wavelengths beyond 1000 nm and facilitate deeper activation (e.g., C1V1, ReaChR).
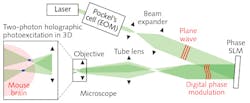
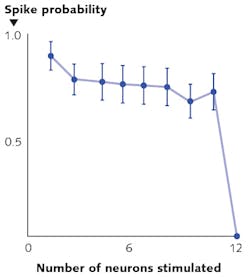
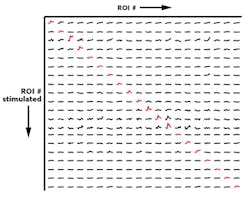
Besides the need for deeper penetration, a key challenge for optogenetics has been to achieve the selective and simultaneous control of large groups of neurons. To overcome this challenge, leading neuroscience research groups are turning to high-peak-power lasers and scanless holographic photoactivation techniques (see Fig. 3). One such example is Dr. Michael Hausser's research group at University College of London (England). "In our work on all-optical interrogation of neural circuits,6 we program a spatial light modulator (SLM) to direct laser beamlets simultaneously to multiple targeted neurons for optogenetic activation in vivo," says Dr. Adam Packer from the Hausser group. "This single-cell level of precision requires two-photon excitation to obtain the necessary spatial resolution in highly scattering brain tissue."REFERENCES
1. T. Hakulinen et al., "Compact fixed wavelength femtosecond oscillators for multi-photon imaging," Proc. SPIE, Multiphoton Microscopy in the Biomedical Sciences XV, 9329, 93290V (Mar. 5, 2015); doi:10.1117/12.2081435.
2. K. Otomo et al., Analyt. Sci., 31, 4, 307-313 (2015); doi:10.2116/analsci.31.307.
3. T. Truong et al., Nat. Methods, 8, 9, 757-760 (2011); doi:10.1038/nmeth.1652.
4. J. Huisken et al., Development, 136, 1963-1975 (Jun. 15, 2009); doi:10.1242/dev.022426.
5. N. Horton et al., Nat. Photon., 7, 205-209 (2013); doi:10.1038/nphoton.2012.336.
6. A. Packer et al., Nat. Methods, 12, 140-146 (2015); doi:10.1038/nmeth.3217.
Julien Klein is senior manager of product marketing for bioimaging applications at Spectra-Physics (Santa Clara, CA); e-mail: [email protected]; www.spectra-physics.com.