OPTOFLUIDICS: Optofluidics assists solar fuel generation
MATTHEW D. OOMS AND DAVID SINTON
Solar energy represents one of the largest possible sources for renewable energy, and circumventing the use of fossil fuels by harnessing solar energy to convert carbon dioxide (CO2) and water (H2O) directly into a fuel—solar fuel—is a global challenge. A variety of approaches are being developed to tackle this challenge of solar fuel generation including photocatalysis of water to produce hydrogen and the processing of biological material for its stored energy content to produce biofuels.
Just as in nature, conversion of CO2 into biologically available carbon or H2O into hydrogen through photochemical reduction in engineered systems using photosynthetic microorganisms or photocatalysts will require the combination of light and fluids interacting on a small scale over large areas. Optofluidics (the marriage of optics and fluidics) is positioned to contribute in this area if current optofluidic approaches toward solar fuel generation are successful and optofluidic tools are developed that enable large-scale energy production.
Why optofluidics for energy?
As a field, optofluidics evolved in the mid-2000s to leverage the unique phenomena that occur when light interacts with matter at the micro- and nanoscales.1 Although a broad range of applications were envisioned, the field has mostly focused on the development of photonic devices useful for sensing, lasing, and optical manipulation with a focus on miniaturization. By combining control over chemical and physical microenvironments and flow conditions with near-field optical enhancements made possible using nanoscale photonic structures, sensing resolution, tunability, and specificity can all be increased while minimizing size.
Recently, the application of optofluidics to energy was proposed for several areas including microalgae photobioreactors, photocatalysis, and fluidic elements to capture and direct light into energy-producing devices.2 In each case, the conversion of solar energy into liquid fuel is enabled through the synergistic application of micro- and nanoscale fluid and light manipulation offered through optofluidic architectures. By leveraging the advantages of control, surface area, and high reaction rates that small-scale fluid and light manipulation offer, paths toward increasing productivity of energy-producing technologies by orders of magnitude have been envisioned.
An example of optofluidic advances that can assist with solar fuel production include using the inherent curvature of liquid interfaces to act as a lens for enhanced sunlight collection. In addition, the high surface-area-to-volume ratio of optofluidic devices can be useful for increasing the reaction rate of photocatalysts used to evolve products such as hydrogen while providing control over the flow of products and reactants. Similarly, biological systems involving photosynthetic microorganisms can benefit from the increased surface areas and short nutrient diffusion pathways inherent in optofluidic systems. This latter approach is particularly promising for solar fuel production.
Photosynthesis through an optofluidic lens
Perhaps the most straightforward route to solar fuel production is to leverage photosynthesis to produce biofuel. The largest energy conversion process on earth is taking place inside the cells of green plants and microalgae inhabiting the biosphere. Chemical reactants and products vary from organism to organism, but central to all photosynthetic processes is conversion of low-energy carbon into higher-energy organic carbon through the addition of energy as photons, which happens at the sub-cellular scale inside of thylakoid membranes of plant and algae chloroplasts and cyanobacteria.
While the science of photosynthesis is rich and diverse, the applicability of optofluidic tools in this area is only beginning to be explored. Developing optofluidic systems incorporating targeted light delivery at cellular length scales has the potential to (1) offer a unique toolset to further our understanding of how organisms, particularly microorganisms, perform this essential function, and (2) open new avenues in solar fuel production using biological organisms.
Conventionally, biological photosynthesis for solar fuel production using microalgae involves large open ponds or tubular structures in which dilute suspensions of microalgae are exposed directly to sunlight. A central issue to these configurations, however, is achieving uniform light distribution to all organisms without compromising overall density of biomass. Invariably, cells near the surface of these ponds or photobioreactors are exposed to light intensities that can be an order of magnitude greater than their saturating limit, causing cellular damage and significantly impacting photosynthetic efficiency.
Conversely, cell-to-cell shading of the organisms occupying the internal spaces of the reactor causes insufficient levels of light and the organic material suffers from sub-optimal energy input. Only a small volume fraction of cells receive optimal light levels at any given time. A consequence of this limitation is that cultivation facilities must use dilute cell suspensions that increase the facility footprint required to produce industrial-scale volumes of biomass. The increasing size of these facilities presents concomitant issues in terms of thermal control required to maintain a viable culture, site selection, and facility maintenance costs.
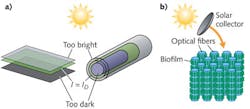
In contrast to these schemes involving bulk cell suspensions, optofluidic architectures can offer light and nutrient delivery on the cellular scale (see Fig. 1). The past year has seen several examples of optofluidic approaches to the cultivation of photosynthetic cyanobacteria that represent precursors to large-scale optofluidic solar fuel production. The uniting feature of all these approaches is the use of evanescent fields to drive photosynthesis within the constituent cells.
The right light
Evanescent fields excited on waveguide surfaces by visible light have penetration depths on the same order as the wavelength—approximately 500 nm. While small, this penetration depth happens to be on the same order as the minor dimension of many types of cyanobacteria, a group of photosynthetic microorganisms. Furthermore, cyanobacteria have their photosynthetic apparati located in membranes around the periphery of the cell walls, within several hundred nanometers from the surface. Cells brought into contact with the surface of a waveguiding substrate will therefore have their photocenters located within the penetration depth of the evanescent light field, allowing excitation of the photosynthetic apparatus by the evanescent optical field (see Fig. 2).By leveraging this synergy between the penetration depth of the evanescent field, the size of the photosynthetic microorganism, and the location of the photocenters within them, a means of targeted cell-scaled light delivery becomes possible. A reactor built on this illumination approach would involve a series of parallel waveguides, each with a surface-adsorbed film of photosynthetic cells. When arranged in a high-density pattern with spacing enough to allow microfluidic transport of nutrients and waste removal, the overall volume fraction of the reactor containing optimally illuminated cells can be orders of magnitude greater than suspensions used in traditional photobioreactor schemes.
The first demonstration of the feasibility of this approach to cell-scaled illumination was published by our group at the University of Toronto and collaborators at Cornell University (Ithaca, NY) earlier this year.3 Evanescent-light-driven photosynthesis was demonstrated using a circular cross-section laser beam incident on the surface of a prism at an angle greater than critical such that the beam underwent total internal reflection. This setup generated an elliptical evanescent field profile on the prism surface and the resulting field decayed exponentially into the medium, confined only to a narrow region above the prism. Cells of wild type Synechococcus elongatus (S. elongatus) were shown to grow preferentially in this field in a manner that is related to optimal radiant light levels for these bacteria with cell-average light intensities that ranged from 12 to 66 W/m2 (see Fig. 3). This method is an alternative to current practices of delivering radiant light to cultures of photosynthetic bacteria by providing a means to confine and control the delivery of energy on the cellular scale.Lab-scale photobioreactors with early measurements of cell growth and photosynthetic productivity have since been described. At the Conference on Lasers and Electro Optics (CLEO) 2012, Pierobon described work toward a scalable photobioreactor using planar waveguides.4 Employing a variety of substrates and light coupling techniques, he showed development of cyanobacterial biofilms on planar waveguides with correlations to evanescent field intensity. In his work, cells of wild type S. elongatus were grown over a planar polymethylmethacrylate (PMMA) waveguide, lit by four high-intensity light-emitting diodes (LEDs). The LEDs had a peak emission at 660 nm, which is well suited for absorption by chlorophyll—a, a primary light-harvesting pigment in the cell. A dilute suspension of S. elongatus was introduced to the surface of the waveguide and the cells were allowed to settle for three days at 35°C in an optically isolated enclosure. Normalized cell surface content was determined from analysis of brightfield microscopy data and found to be on the order of 1 × 109 cells per square meter.
Jung et al. recently described evanescent growth of cyanobacteria on a slab waveguide.5 In her study, she showed that the photon usage efficiency using evanescent illumination of cell cultures was comparable to that of directly illuminated cultures. Cultures were grown on a glass cover slip that served as the waveguide. Light from a 660 nm laser diode was coupled into the cover slip and the light intensity of the beam exiting the cover slip was measured. By monitoring the change in output power over the course of several days while the cells proliferated, the energy absorbed by the film was determined and correlated to the number of cells, as determined by time-series microscope images of the cover slip surface. By assessing the cell density achieved using the slab waveguide, Jung estimated a twelve-fold improvement in volumetric productivity for a photobioreactor based on parallel slab waveguides compared to conventional photobioreactor designs.
The application of optofluidics to biofuel production is an emerging area of interest with particular benefits for operational energy density and concomitant benefits for thermal control, site selection, and containment. By using near-field optics to focus light where it is needed at the photosynthetic membranes of energy-producing microorganisms, new architectures for mass cultivation of organisms in facilities with small-scale footprints (and correspondingly simplified thermal control) are possible. Pairing this type of optofluidic architecture with advances in genetically engineered organisms designed to evolve fuels directly into the surrounding media could enable large-scale fuel production in the not-too-distant future.
REFERENCES
1. D. Psaltis, S.R. Quake, and C. Yang, Nature, 442, 7101, 381–386 (July 2006).
2. D. Erickson, D. Sinton, and D. Psaltis, Nature Photon., 5, 10, 583–590 (September 2011).
3. M.D. Ooms et al., Physical Chemistry Chemical Physics: PCCP, 14, 14, 4817–4823 (April 2012).
4. S. Pierobon et al., "A Scalable Evanescent Light-based Photobioreactor - OSA Technical Digest (online)," in CLEO: Science and Innovations, paper CW1G.4, San Jose, CA (2012).
5. E.E. Jung et al., "Slab waveguide photobioreactors for microalgae based biofuel production," accepted paper in Lab On A Chip (2012).
Matthew D. Ooms is a doctoral student in the Sinton Lab and David Sinton is associate professor, Mechanical and Industrial Engineering department, and director, Centre for Sustainable Energy, at the University of Toronto, 5 King's College Rd., Room MC226, Toronto, Ontario M5S 3G8, Canada; e-mail: [email protected]; http://sintonlab.mie.utoronto.ca and http://cse.utoronto.ca.