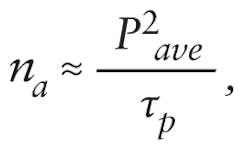MULTIPHOTON MICROSCOPY: Turnkey femtosecond lasers fuel growth of multiphoton imaging
ARND KRUEGER
Since the first report of two-photon excited laser scanning fluorescence microscopy in 1990, the use of multiphoton imaging in biomedical research has seen tremendous growth, fueled by the advent of all-solid-state ultrafast lasers. Compared to conventional confocal microscopy, multiphoton imaging methods offer deeper tissue penetration, less cell damage, and inherent three-dimensional (3D) optical sectioning. Multiphoton microscopy is used in many fields of biomedical research, where the capability to image deeply, in vivo, and with higher sensitivity is of particular interest.
Two-photon excited fluorescence microscopy
In conventional confocal laser scanning microscopy, the fluorescence excitation source is a tightly focused continuous-wave (CW) laser beam. The laser beam waist is re-imaged onto a pinhole (the confocal aperture), which prevents fluorescence generated outside the focal plane from reaching the detector. Measurement of the fluorescence intensity enables a 3D image to be constructed point by point as the laser spot or the sample stage is sequentially scanned in all three dimensions. The spatial resolution of the technique is close to the diffraction limit of the excitation wavelength.
Conventional confocal microscopes are essential tools in today's biomedical research laboratories, but they do have limitations. Out-of-focus excitation, which does not contribute to the 3D image, increases the potential of damage to living specimens–and the visible and ultraviolet wavelengths optimal for direct excitation do not penetrate deeply into tissue. Furthermore, re-imaging the fluorescence from the focus of the laser beam onto the confocal aperture generates losses and reduces sensitivity. These factors limit the ability to image living cells and deeply into tissue.
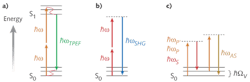
In 1990, Denk, Strickler, and Webb at Cornell University (Ithaca, NY) showed that these limitations could be avoided by using modelocked, femtosecond lasers for two-photon excitation of fluorophores (see Fig. 1a).1 Although this nonlinear process has a low probability, the peak power of femtosecond pulses (typically at 80 MHz repetition rate) together with the high-NA (numerical aperture) microscope objective generate photon densities within the focal volume that are enough to drive two- (and even three-) photon absorption. Elsewhere in the sample, the power density is too low for nonlinear excitation, so there is an inherent spatial sectioning capability that obviates the need for a confocal pinhole. This means the detector can be located close to the specimen, resulting in increased sensitivity and signal-to-noise ratio. There is also virtually no out-of-focus excitation so cell and tissue damage is greatly reduced. Moreover, because multiphoton imaging uses longer wavelengths where there is less absorption and scattering, the two-photon technique can penetrate much farther into tissue (hundreds vs. tens of micrometers).
These advantages for live-cell and deep-tissue imaging resulted in two-photon excited fluorescence microscopy being quickly embraced by researchers in cell biology and the neurosciences. It was the advent of compact, fully automated, and widely wavelength-tunable femtosecond Ti:sapphire lasers that triggered explosive deployment around the globe. Today, these lasers deliver pulses as short as 70 fs and peak powers as high as 450 kW over a tuning range that exceeds 300 nm, allowing the user to select the optimum excitation wavelength for the fluorophore.
The latest advance in laser sources is the integration of automated dispersion compensation, which counteracts pulse broadening caused by the positive dispersion of the microscope setup. Femtosecond pulses have a wide spectral bandwidth (~7 nm for 100 fs pulses at 800 nm). As they travel through dispersive optics, the wavelength components within that bandwidth progress at different speeds, spreading the pulse out over time. Most dispersion can be attributed to the high-NA objective and acousto-optical modulator, which is integrated in most commercial multiphoton instruments for attenuation and as a rapid beam block.
Depending on system configuration, pulse broadening can be severe, stretching a 100 fs pulse from the laser to several hundred femtoseconds at the sample. This reduces excitation efficiency and image brightness. The number of photons absorbed per fluorophore per pulse, na, is inversely proportional to pulse duration. For a given dye, excitation wavelength, and pulse repetition rate, na is given by:
where Pave is the average laser power and τp is the pulse duration at the specimen.1
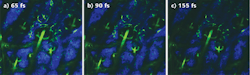
The amount of fluorescence can be increased by raising the average power, but that heightens the potential for tissue damage. Minimizing the pulse duration by counteracting the microscope dispersion is the best way to optimize image brightness and tissue penetration depth. Dispersion compensation significantly improves the signal-to-noise ratio in multiphoton microscopy (see Fig. 2).2As a result of these benefits, lasers that integrate automated dispersion compensation into the Ti:sapphire laser have become ubiquitous in today's state-of-the-art two-photon microscope systems. Compact and easy to integrate, they automatically minimize the pulsewidth delivered to the sample at any wavelength within their tuning range. Beam pointing is actively stabilized, which is critical since any beam shift or tilt would compromise the integrity of the instrument and the experiment.
Wavelength extension and multimodality
Two-photon excited fluorescence microscopy is the most commonly used multiphoton imaging modality. Second (and third) harmonic generation is generally available from the same equipment. There are other multiphoton techniques that require more than one input wavelength, including coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering microscopy. These offer the same inherent 3D optical sectioning capability, and both are label free–that is, they do not require fluorophores. Instead, they exploit intrinsic properties of the specimen for contrast generation.
Coherent anti-Stokes Raman scattering is a four-wave mixing process of the so-called pump (P), Stokes (S), probe (P'), and anti-Stokes (AS) waves (see Fig. 1c). To simplify the setup, pump and probe are typically set at the same frequency (wavelength) and originate from the same laser beam (ωp=ωp',λp=λp'). If for molecules within the focal volume the frequency difference between the pump and Stokes waves matches a molecular vibration (ΩV = ωP - ωS), resonant enhancement creates a collimated, directional anti-Stokes beam at higher energy (ωAS = 2ωP - ωS). The intensity is typically several orders of magnitude higher compared to the corresponding signal measured in conventional Raman spectroscopy.
As a third-order process, CARS microscopy requires ultrafast laser pulses that deliver high peak intensities. It also requires two pulse trains that have different wavelengths (λP = λP' and λS), but are synchronized in time. They must be focused and overlapped in space and time, and scanned together within the specimen. The anti-Stokes signal is measured pixel by pixel and used to establish the CARS image.
The laser sources commonly used for CARS are optical parametric oscillators (OPOs), which convert an ultrafast pulse train into two tunable ones of longer wavelengths called signal and idler. All three pulse trains are inherently synchronized and can, with proper control of the delays, be focused to coincide in space and time. For CARS microscopy, pulse durations of a few picoseconds are optimal, as the narrower spectral bandwidth matches the spectral width of most Raman lines, reducing the nonresonant background. Some biologically significant vibrations, like the C-H2 stretching modes in lipids at ~2850 cm-1, have a broader spectral profile and enable good signal-to-background ratio when probed with femtosecond pulses.3
One advantage of femtosecond pulses is multimodality–the ability to combine CARS microscopy with, for example, two-photon excited fluorescence and SHG imaging, both of which benefit from shorter pulsewidths. The ideal source for multimodal microscopy, including femtosecond CARS, is a Ti:sapphire laser-pumped OPO, such as the Spectra-Physics Inspire. End users can use the Ti:sapphire tuning range for two-photon excited fluorescence and SHG imaging. The fully automated OPO provides output in the IR beyond 1100 nm and extends the reach to longer wavelengths. The combination of pump laser and OPO delivers the fully synchronized pump and Stokes pulse trains that enable femtosecond CARS for a range of frequencies ΩV, including the C-H2 stretching vibrations.
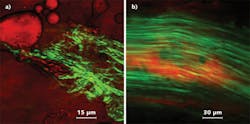
Another method for generating the Stokes beam in CARS microscopy involves supercontinuum generation in a photonic crystal fiber (PCF). This was demonstrated in research laboratories as early as 2003.4, 5 Last January, Newport released a wavelength extension unit, which, in combination with a femtosecond Ti:sapphire laser, provides users with an integrated, supercontinuum-based solution for broadband CARS microspectroscopy and imaging.6 A beamsplitter routes ~20% of the 800 nm input beam through a Faraday isolator and a variable attenuator into a PCF, where the supercontinuum is generated through nonlinear processes. The dispersion profile of the PCF generates a spectral density peak at ~1040 nm that, with appropriate spectral filtering, serves as the Stokes pulse train. This is recombined with the residual 800 nm input from the femtosecond laser, which serves as the pump (and probe) wave–enabling CARS imaging of lipids using the CH2 vibrations at ~2850 cm-1. The components used to deliver the pump and Stokes pulses collinearly and overlapped in time are integrated in the device. Combined with a tunable ultrafast laser the setup enables multimodality: The full Ti:sapphire tuning range can be accessed through the pump beam path for two-photon excited fluorescence and SHG microscopy; CARS imaging can be done with the input wavelength set at 800 nm (see Fig. 3). Although the Stokes beam power density is lower than that typically generated by an OPO, supercontinuum-based CARS provides a cost-effective, compact upgrade for many two-photon microscope systems installed in laboratories.
REFERENCES
1. W. Denk, J.H. Strickler, W.W. Webb, Science, 248, 73-76 (1990).
2. J. Field et al., Opt. Exp., 18, 13, 13661-13672 (2010).
3. H. Chen et al., Opt. Exp., 17, 3, 1282-1290 (2009).
4. H. Paulsen et al., Opt. Lett., 28, 13, 1123-1125 (2003).
5. H. Kano, H. Hamaguchi, Appl. Phys. Lett., 86, 121113-121115 (2005).
6. Newport Application Note 36, Newport Technology & Applications Center (2007).
Arnd Krueger is senior strategic marketing director at Newport Spectra-Physics, 3635 Peterson Way, Santa Clara, CA 95054; e-mail [email protected]; www.newport.com.