MEDICAL IMAGING: OCT finds value in both art and science
LAURIE MORGUS
Although the term “optical coherence tomography” (OCT) is a familiar one to optics and photonics professionals, the technology itself is complex and specialized enough that even many people skilled in photonics don’t know much about it. Most recognized as a medical tool, OCT is useful in other areas as well. A review of its workings, as well as some areas of application, can serve to demonstrate its wide potential.
Optical coherence tomography is a noninvasive, real-time imaging technique that is capable of providing one-dimensional (1D) depth, 2D cross-sectional, and 3D volumetric images of nontransparent scattering media with micron-level resolution and millimeters of imaging depth. Fourier-domain OCT is based on low-coherence interferometry, which uses a light source with low temporal coherence to measure optical-path-length delays in a sample. To obtain cross-sectional images with micron-level resolution, an interferometer is set up to measure optical-path-length differences between light reflected from the sample and reference arms.
Swept-source OCT (SS-OCT) and spectral-domain OCT (SD-OCT) differ in how they measure the OCT interferogram. Spectral-domain OCT systems do not have any moving parts and therefore offer high mechanical stability and low phase noise. Availability of a broad range of CCD cameras has also enabled development of SD-OCT systems with varying imaging speeds and sensitivities. In contrast, SS-OCT systems use a frequency-swept light source and photodetector to rapidly generate the same type of interferogram. Due to the rapid sweeping of the laser source, high peak powers at each discrete wavelength can be used to illuminate the sample to provide greater sensitivity with little risk of optical damage.
Use in biology and medicine
The optical analog of ultrasound, OCT provides significantly higher resolution than ultrasound at the expense of imaging depth. With the ability to image up to 6 mm in depth and to achieve better than 5 μm of axial resolution, OCT fills a niche between ultrasound and confocal microscopy. The noncontact, noninvasive nature of OCT is particularly suited to biological studies of tissues and small animals. Recent OCT advances have yielded systems that are capable of high-speed imaging at rates in excess of 100,000 lines per second.
Optical coherence tomography has been used in a multitude of biological applications, including pharmaceutical research aimed at monitoring coating thicknesses and uniformity, functional neuroimaging, studies of the microvascular system, studies of the macroscopic configuration and real-time growth and migration of biofilms, and noncontact imaging of human vocal folds.1-5 In many instances, the OCT imaging technique could have a profound impact on current medical procedures. For instance, the current gold standard for confirming early detection of abnormal tissue growth on the larynx is to conduct an excisional biopsy, which is highly invasive, requires anesthesia, and can potentially cause irreversible hoarseness. In contrast, by combining the OCT technique with videolaryngoscopy, it is possible to detect superficial and subsurface lesions on the larynx during an in-office examination, without the need for anesthesia or direct contact with the neck tissue.
Imaging the kidney
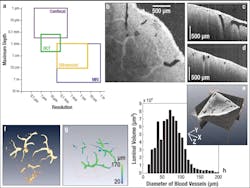
Another biological imaging application was recently carried out by researchers from the University of Maryland (College Park, MD) and Georgetown University (Washington, DC). They used a Thorlabs SS-OCT system for high-speed, real-time 3D visualization and volumetric rendering of dimensional changes in renal blood vessels and other tubular structures. With an imaging depth of up to 800 μm in renal tissue and the ability to scan the entire organ quickly, OCT can provide a global in-situ assessment of renal blood vessels, tubules, and glomeruli. Such information on tubular morphology has been linked to kidney-donor viability, making OCT a promising candidate for clinical renal evaluation prior to transplant.
During the experiment, 3 × 3 × 2.25 mm images of a human kidney (512 × 512 × 512 pixels) were obtained in vitro. From these, the diameters of blood vessels could be determined automatically via image processing. Because the various tubular structures within the kidney (such as blood vessels, tubules, and glomeruli) exhibit different backscattering intensities, an automated image-processing method was used to isolate the different regions of interest (ROI). The boundary and skeleton were determined for each ROI, thereby enabling the calculation of the diameters of each luminal position based on the shortest skeletal-boundary distances. Cross-sectional OCT images of the human kidney were obtained in the X-Y, X-Z, and Y-Z planes as well as a composite 3D view (see Fig. 1). Although the various kidney vascular networks are distinguishable in all three cross-sectional images, segmentation was used to isolate the renal blood vessels to determine their luminal diameters; then 3D visualization software was used to reconstruct the blood vessel images.
Seeing the structure of art
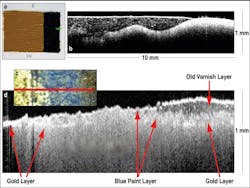
In addition to biological applications, the use of OCT imaging has also extended to nonbiological studies; for example, OCT has been used for noninvasive examination of the stratigraphy of historically significant paintings,6-8 as well as to detect defects in harvested onions prior to, during, and after postharvest storage.9 In the former case, a Thorlabs Fourier-domain OCT system was used to examine Saint Catherine of Alexandria with a Donor, painted between 1480 and 1500 by Pintoricchio and currently housed in the National Gallery in London (National Gallery No. 693).As a first step in the experiment, a test panel was prepared using a traditional ground layer of chalk mixed with rabbit-skin glue; then a layer of smalt in linseed oil was painted on top of the base layer (see Fig. 2). In the corresponding OCT image, the interfaces between the various layers are clearly visible, as are the smalt particles, which appear as bright dots. Because the OCT system monitors the intensity of the backscattered light, larger amounts of backscatter correspond to bright spots in the image; backscattering is most evident at medium boundaries due to the change in refractive index. When a portion of the painting is matched to a corresponding OCT image, the OCT image is seen to display an interesting layered structure of varnish, blue-hued paint (most likely ultramarine), and gold. The particular subsection of the photo was chosen because it contains three distinct regions: one where there is only a gold background, one where all three layers are present, and one where the varnish had been removed. The OCT image clearly depicts all three regions. Due to the fast acquisition speeds of OCT and its ability to distinguish multiple surface layers, this method holds promise for monitoring varnish-cleaning processes.
REFERENCES
1. Y. Lan et al., “A Novel Non-Destructive Optical Method in Pharmaceutical Research Using Optical Coherence Tomography,” poster pres. at the AAPS Meeting (November 2008).
2. Y. Chen et al., J. Neurosci. Methods, 178, 162–173 (2009).
3. A. Mariampillai et al., Optics Lett., 33, 1530 (2008).
4. M. Wagner et al., “Potential successors of confocal laser scanning microscopy for the three-dimensional visualization of biofilms,” poster pres. at the Biofilms III Conf. (October 2008).
5. H. Wisweh et al., Optical Coherence Tomography and Coherence Techniques IV, 7372, 737201 (2009).
6. H. Liang et al., “A. Non-Invasive Imaging of Subsurface Paint Layers with Optical Coherence Tomography,” Conservation Science 2007, Non-destructive Testing, 171–176.
7. M. Spring et al., “Optical Coherence Tomography–A Tool for High-Resolution Non-Invasive 3-D Imaging of the Subsurface Structure of Paintings,” Proc. ICOM-CC Triennial Conf., Vol. II, 916–923 (2008).
8. H. Liang et al., “Optical Coherence Tomography for Art Conservation & Archaeology,” Proc. SPIE, 6618, 661805 (2007).
9. I.V. Meglinski et al., Laser Physics Lett., 7, 169–176 (2010).
Laurie Morgus is the head of marketing communications at Thorlabs Inc., 435 Route 206, Newton, NJ 07860; e-mail: [email protected]; www.thorlabs.com