PHOTONIC FRONTIERS: OPTICAL COHERENCE TOMOGRAPHY: Complementary techniques enhance the power of OCT
Invented two decades ago, optical coherence tomography (OCT) is carving out a growing niche in biomedical imaging for diagnosis and research. OCT’s strengths include its high resolution for tissue imaging, its noninvasive nature, and its use of innocuous near-infrared (NIR) radiation. Those are offset by its insensitivity to molecular composition and a penetration depth limited to about 3 mm by scattering and absorption in most tissue. Nonetheless, writes Rainer A. Leitgeb of the Medical University of Vienna (Vienna, Austria) in a review paper, “OCT could be seen as the missing link between cellular imaging on the one hand and tissue and organ imaging on the other,” adding a vital capability to medical imaging.1
So far, OCT has been most successful in ophthalmology, where the eye’s transparency allows light to penetrate all the way to the back of the eye and enables examination of the retina with high resolution. Another fast-growing application is cardiology, where fibers threaded through coronary arteries allow OCT to examine arterial plaque with unprecedented clarity. Many other applications are emerging, and developers are working on instruments that combine OCT with other diagnostic techniques, such as fluorescence and Raman measurements. This article will give a brief overview of an extremely active field.
OCT basics
Optical coherence tomography is an optical analog of ultrasound imaging, which builds images by recording soundwaves scattered by tissue. OCT collects image data by scanning a sample and combining the backscattered light with a delayed reference beam in an interferometer. Using a light source with limited coherence keeps multiply scattered photons out of phase with the reference beam at the detector, so only directly scattered light is recorded. Contrast in the image arises from changes in the refractive index within the tissue.
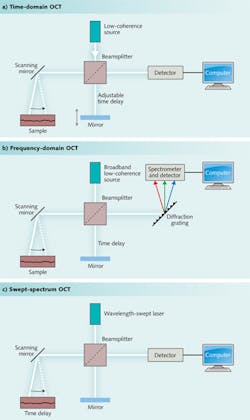
Data can be collected in one of three ways, shown in Fig. 1. The first approach to be developed was time-domain measurements, made by adjusting the length of the reference beam in the interferometer to measure scattering at different depths. Although time-domain systems are still used, most measurements now are made by frequency (or Fourier) domain instruments. One class of frequency-domain instruments uses a fixed broadband source, and directs the combined scattered light and reference beam to a spectrometer, forming a spectrum that is recorded and analyzed on a computer. A second uses a light source that repeatedly scans across its output spectrum and focuses the combined beams onto a single detector to produce a frequency-swept spectrum. Many variations exist, including systems that scan with multiple beams to measure tissue at different depths.
In addition to detecting refractive-index differences within tissue, OCT also can detect anything else that affects the phase, amplitude, or polarization of light within the imaged tissue. For example, monitoring the polarization of light within tissue can reveal changes in the condition of collagen, the most common protein in mammals, which is naturally birefringent. Recording a series of frequency-domain OCT images can determine the Doppler shift of blood flowing through the tissue.
Although we think of most tissue as opaque, it is translucent in the red and NIR spectra, allowing OCT to penetrate thin layers. Penetration depths typically are 1–3 mm at 800–1300 nm. Tissue is water to a first approximation, so the 1400 nm water band sharply limits penetration depth, although OCT can penetrate useful depths in the 1550 nm band. The shorter wavelengths in the 800–1300 nm band have the best contrast, but their penetration is limited to shallower depths than the longer wavelengths. Thus the best wavelength depends on the application.
Broadband light sources provide the short coherence length needed for OCT. Lamps have been used in some cases, but the primary sources are pulsed or tunable broadband lasers, superluminescent diodes (which amplify spontaneous emission but lack resonators), and supercontinuum sources. Frequency-swept OCT requires lasers that can emit a narrow line while being tuned quickly across a broad band. Powers typically range between 1 and 100 mW, but the higher end of the range is not used in ophthalmology. Frequency-swept sources may use MEMS scanners or other types of moving mirrors to scan the spectrum.
Technology advances have driven the advance of OCT. Improvements in light sources and detectors have increased speed, resolution, and detection sensitivity. Leitgeb writes that scanning speed has increased a factor of 10 million since the first OCT scanner was demonstrated in 1991. With further enhancements in resolution, he adds, OCT in “the near future could provide real-time in situ biopsy with cellular resolution,” reducing false positives and improving the precision of treatment.
Ophthalmology and other applications
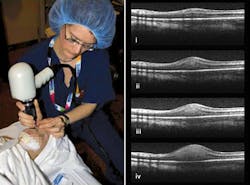
“OCT and the eye is a match made in heaven,” David Huang of the Oregon Health & Science University (Portland, OR) told SPIE’s Photonics West in January. The eye’s transparency and the importance have made ophthalmology the most successful OCT application (see Fig. 2). Although ophthalmologists still use slit lamps for standard initial exams, they turn to OCT for 90% of “serious” retinal exams, says Eric Swanson, a serial entrepreneur who edits OCT News (http://www.octnews.com). OCT has performed some 100 million ophthalmological scans, he says, and allows nonspecialists to diagnose eye disease before the damage becomes untreatable.Most procedures now involve the retina. Three-dimensional imaging with 5 µm resolution is now standard with OCT, allowing monitoring of the abnormal blood vessels involved in age-related macular degeneration. The condition was “previously pretty much untreatable,” Huang told SPIE, but OCT tells ophthalmologists when they need to inject drugs that control the blood vessels, limiting the damage to vision. High-resolution imaging of the retina also can spot early signs of glaucoma, and monitor blood flow in the underlying choroid as well as in the retina.
Imaging of the cornea also is growing rapidly. OCT can measure the topology of the cornea and the structure of its internal layers, which are important for optimizing results of refractive surgery. Ophthalmologists also are studying ways that OCT could be used in the operating room, in treating such conditions as corneal scars and performing a new cataract surgery that uses femtosecond lasers to help remove hardened lens material.
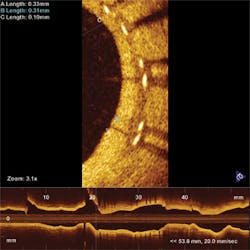
Another hot OCT application is cardiology, in which singlemode fibers are inserted into arteries and helically scanned to examine plaque (see Fig. 3). The goal is to find vulnerable plaque, which is at high risk of detaching without warning and causing potentially fatal thrombosis. OCT is the only technology capable of assessing such plaque, says Andrew Rollins of Case Western Reserve University (Cleveland, OH). His group is focused on developing tools to analyze and visualize the vast amounts of data collected to aid physicians in treating vulnerable plaque while patients are being examined. “If OCT is to be used in making a decision, it has to provide feedback instantly,” says Rollins. He also is developing OCT tools to examine the developing embryonic heart, to help researchers understand and treat congenital heart defects.Researchers also are investigating OCT applications in cancer detection and treatment in the gastrointestinal and pulmonary systems and the reproductive tract. Imaging is performed through an endoscopic probe, looking for changes in morphology that might indicate cancer risks. “The gastrointestinal tract is one of the biggest commercially,” says Swanson. Esophageal disease is rare but tends to be very deadly, so effective early imaging could be a lifesaver.
Breast cancer is a tougher problem. A recent study shows polarization-sensitive OCT can identify cancerous breast tissue.2 However, the bulk of breast tissue is deeper than the 1–3 mm limit of current OCT imaging.
Dermatology might seem a natural market but imaging through skin is difficult, and it’s easy to remove surface lesions for conventional biopsies, which yield more definitive results. From a dermatologist’s viewpoint, Thilo Gambichler of Ruhr University (Bochum, Germany) wrote in a recent view, the most promising approach is combining standard OCT with Raman, Doppler, and fluorescence techniques that yield optical data beyond pure backscattering.3
Looking forward
The ongoing OCT boom is evident in the dozens of papers scheduled for the Optical Coherence Tomography and Coherence Domain Optical Methods in Biomedicine conference—the 16th in a series—at SPIE Photonics West 2012 next month. It’s also evident in the installation of some 17,000 systems for ophthalmology alone through 2009, Huang said at this year’s Photonics West.
Yet much remains to be done. Integrating complementary optical techniques will help overcome OCT’s inability to directly sense molecules, so systems can better diagnose tissue composition. Better data processing and visualization is needed to provide the real-time imaging required during delicate microsurgery. And penetration depth remains a stubborn problem. “The Achilles heel of OCT has been that you can’t get to 1 cm,” Swanson says. He won’t say it’s impossible to solve, but he doesn’t know how...yet.
REFERENCES
1. R.A. Leitgeb, “Current Technologies for High-Speed and Functional Imaging with Optical Coherence Tomography,” Adv. in Imaging and Electron Phys., 168, 109–192 (2011); doi:10.1016/B978-0-12-385983-9.00003-X.
2. Y. Verma et al., “Imaging of Human Breast Tissue Using Polarization Sensitive Optical Coherence Tomography,” Laser Phys., published online (Oct. 3, 2011); doi:10.1134/S1054660X11210225.
3. T. Gambichler, V. Jaedicke, and S. Terras, “Optical coherence tomography in dermatology: technical and clinical aspects,” Arch. Dermatol. Res., 303, 457–473 (2011); doi:10.1007/s00403-011-1152-x.
About the Author
Jeff Hecht
Contributing Editor
Jeff Hecht is a regular contributing editor to Laser Focus World and has been covering the laser industry for 35 years. A prolific book author, Jeff's published works include “Understanding Fiber Optics,” “Understanding Lasers,” “The Laser Guidebook,” and “Beam Weapons: The Next Arms Race.” He also has written books on the histories of lasers and fiber optics, including “City of Light: The Story of Fiber Optics,” and “Beam: The Race to Make the Laser.” Find out more at jeffhecht.com.