Editor's Note: This article is adapted and updated from a review paper,1 with permission from IOP Publishing.
Optical coherence tomography (OCT) is used mainly to depict structural characteristics. But this versatile imaging technique is also able to provide functional imaging of live, intact tissue, and in recent years, researchers have increasingly pursued extensions to realize this potential. These extensions expand OCT's relevance for clinical work by providing richer information about such dynamics as blood flow, collagen organization, and oxygenation.
This article, a summary of an extensive and fully referenced review,1 examines five such extensions from the vantage point of clinical applications (see table).
Doppler OCT
Perhaps the most widely used functional OCT approach, Doppler OCT (DOCT), uses temporal variations in index of refraction to enhance information provided by inherent contrast. It measures the frequency shift of light scattered by a moving particle to determine the particle's speed.
Although additional computer resources may be needed for processing (especially to depict flow in real time), the only adaptations necessary for implementing DOCT on standard equipment involve signal processing. And signal processing requirements depend on the data acquisition approach—whether time- or Fourier- (aka frequency) domain.
Time-domain (TD-OCT) involves a moving mirror in the reference arm, which centers the interference signal on a fixed Doppler frequency. Coherent demodulation, with a lock-in amplifier set to this frequency, enables detection of interference fringes produced by light scattered from the specimen. By contrast, Fourier (frequency) domain (FD-OCT) acquires data from the whole sample depth simultaneously with a fixed path length in the reference arm. Typical DOCT implementation involves acquiring successive axial scans (A-scans) at a specific lateral location, and calculating the phase difference between them—which correlates to the speed of the target within the sample.
It is true that time varying signals generated by bulk motion (as are typically seen in biological samples) can confuse the true Doppler signal, though there are ways to minimize this effect. And, researchers have devised ways to determine the angle between the OCT beam and the direction of the velocity—which is necessary for accurate velocity measurement in all modes of Doppler imaging.
Variations
DOCT variations include optical angiography (OAG), which uses SD-OCT with modulation in the reference arm path length. This method has enabled imaging of blood perfusion in a living adult mouse with higher signal-to-noise ratio (SNR) than phase-resolved DOCT.
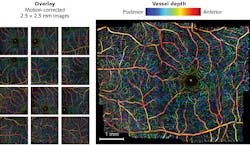
Researchers have also used phase variance as a contrast mechanism to distinguish between moving and fixed scatterers to demonstrate speckle-variance OCT. This technique can provide greater sensitivity than Doppler for angles of incidence approaching 90°. Variance processing methods in FD-OCT have enabled depth-resolved visualization of capillary beds in the retina (see image at top of this page).
While its primary application has been in small animal research—for instance, to image and analyze heartbeat and blood flow in live rat embryos at single-cell resolution (using a swept-source), and to measure shear stress in the developing hearts of quail embryos (with 3D OCT over time)—DOCT has shown potential for multiple clinical uses in ex vivo or in vivo human studies by observing blood flow modulation. Though not yet actively in clinical use, it promises significant benefit for diagnosing disease and monitoring treatment response.
In ophthalmology, DOCT can potentially monitor progression of pathologies such as diabetic retinopathy and glaucoma. Endoscopic DOCT enables examination of such gastrointestinal processes as esophageal varices and neoplasms, achieving a velocity resolution of ~100 μm/s, a 10-100X improvement over Doppler endoscopic ultrasound.
In solid organs, DOCT has been used to study kidney microcirculation in both animals and humans, as well as other anatomical sites in humans. It has been shown to accurately measure blood flow with both micron-level spatial resolution, and velocity resolution up to 10 μm/s. Computational methods can remove bulk-motion artifacts and precisely determine the angle between the illumination beam and direction of flow.
Thorlabs (Newton, NJ) offers Doppler OCT as an option on both spectral-domain (SD) and swept-source (SS) systems. Optovue's (Fremont, CA) AngioVue OCT system for 3D visualization of retinal vasculature was FDA approved in February 2016.
Polarization-sensitive OCT
PS-OCT collects light resolved by polarization and thus reveals birefringence, which can provide contrast in samples with a high degree of microscopic structural order, including cartilage, teeth, and blood vessel walls. Structural changes associated with certain disease processes cause changes in birefringence.
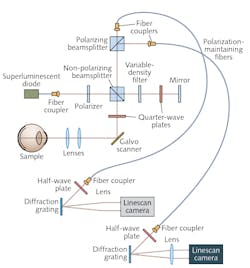
Because it involves collection of signals at more than one polarization, PS-OCT requires complexity in system design and collection schemes. Data from each polarization channel are typically processed independently until the final stage when the images are analyzed to provide contrast. Early methods for collecting multiple polarizations, based on TD-OCT, advanced to include 3D PS-OCT. FD-OCT boosts imaging speeds, thanks to improved SNR (see Fig. 1).In SS-OCT, collection optics typically consists of a single detector or a pair of detectors in a balanced configuration-so the PS implementation is similar to that used in TD. Interferometers for SS-OCT are typically fiber-based: Polarization-maintaining fibers preserve a signal's polarization dependence. Adding a circulator and two fiber-coupled polarizing beamsplitters enables balanced detection (this requires four detectors, and suppression of the opposite polarization will be limited by the beamsplitters' extinction ratios).
PS-OCT has been applied to study the eye, skin, esophagus, breast tissue, cartilage, and teeth. In an eye study, it showed reasonable correspondence with manual grading of color fundus photographs, which is the current gold standard for diagnosis.
PS-OCT has also been helpful for studying collagen, whose structure transitions from a rod-like alpha helix to a random coil conformation after heating; reduced birefringence is a hallmark of burned tissue.
Other prospective applications include assessment of cartilage organization, and determination of dental caries (because dental enamel contains birefringent hydroxyapatite crystals).
Recent demonstrations have achieved high spatial resolution and accuracy in determining the phase difference between orthogonal polarization states. This additional structural information can be leveraged to diagnose disease, evaluate injury, or monitor therapeutic response.
Optical coherence elastography
Disease can alter the elastic properties of tissue, as tumors are often stiffer than the normal tissue surrounding them and edema, fibrosis, and calcification changes the elastic modulus of the extracellular matrix.
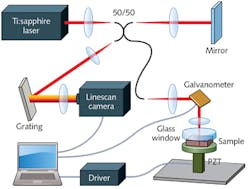
Elastography, which combines a known mechanical modulation with imaging, can quantitatively measure tissue strain and elasticity for disease detection. The approach was first developed using ultrasound (sono-elastography) imaging, and later with MRI. The more recent combination of sono-elastography with OCT—which provides greater resolution—produced optical coherence elastography (OCE), which has evolved to not only differentiate tissue types (see Fig. 2), but also to produce 3D imagery, and, integrated with a needle biopsy probe, to image deeper into tissue.Elastography is currently used to examine breast, brain, and lung tissue for malignancy, and to assess atherosclerotic plaques within arteries. Intravascular ultrasound elastography can extract arterial radial strains at a spatial resolution of 200 μm, but vulnerable atherosclerotic plaques have structural elements as small as 50 μm. Intravascular OCE promises high-resolution characterization of strain 1.0-1.5 mm below the vessel surface—the region most susceptible to plaque disruption. Intravascular OCT is able to resolve thin cap fibroatheromas (TCFA), the intracoronary plaques thought to be responsible for acute coronary syndrome. By detecting changes in mechanical properties because of TCFA formation, OCE may be able to predict plaque rupture.
Though OCE has not been widely embraced for clinical use, research indicates great potential. It can identify intravascular blood clots, and has been evaluated for such clinical applications as eye, skin, and breast cancer, as well as cystic fibrosis.
Spectroscopic OCT
Wavelength-dependent absorption and scattering of light can be used to extract health-related functional parameters such as blood oxygen saturation. Spectroscopic OCT (SOCT) can measure both.
As with conventional OCT, SOCT measurements can be made in either the time or frequency domain. The detected interferometric signal can be digitally processed to construct a time-frequency distribution containing both spatial (time) and spectroscopic (frequency) information. The typical approach is to construct a spectrogram using the short-time Fourier transform (STFT). In the case of a time-domain signal, a temporal (depth) window is applied and a Fourier transform performed to determine the signal's spectroscopic content at a particular depth. This operation is repeated at various depths to construct the SOCT image.
While STFT involves an inherent tradeoff between spatial and spectral resolution, a new development, the dual window (DW) method, calculates the product of two STFTs. One STFT generates high depth resolution and a second generates high spectral resolution, without introducing the artifacts commonly seen with other signal processing methods. While DW cannot achieve STFT's true spectral fidelity for analyzing bulk tissue properties, it stands out in applications such as cell nuclei morphology assessment for early cancer detection, where STFT's spatial averaging obscures the desired signal.
SOCT has been shown to measure blood oxygen saturation by leveraging the distinct absorption features of oxy- and deoxy- hemoglobin. In carcinogenesis studies using animal models, DW has proven able to detect significant differences in the cells of normal tissue vs. precancerous lesions.
Cardiovascular medicine could benefit greatly from SOCT's ability to noninvasively detect cholesterol plaques—which could avoid invasive procedures such as coronary catheterization.
Finally, a study that used SOCT in vivo in a mouse model demonstrated its diagnostic utility for qualitatively differentiating healthy and burned tissues.
For successful translation to the clinic, however, development is required-first, to separate scattering and absorption coefficients, and second, to improve the accuracy of hemoglobin concentration and oxygen saturation measurements through careful selection of relevant properties (e.g., illumination wavelength, illumination power, and measurement time) and processing routines.
Molecular imaging in OCT
Unlike other medical imaging modalities, OCT is not inherently able to track molecular species. Since it relies on coherent detection of photons that have interacted with a sample, OCT cannot directly detect incoherent fluorescent photons and instead must detect them interferometrically or by observing absorption following excitation. Another source of molecular contrast for OCT comprises exogenous agents designed to produce unique scattering features.
Early molecular imaging OCT used spectroscopic techniques to detect absorption of near infrared (NIR) dyes—an approach complicated by scattering. Later development of a triangulation method to detect indocyanine green (ICG) proved able to distinguish the contrast agent from other spectral features by obtaining high-resolution spectra using DW.
Another method, pump-probe OCT (PPOCT), uses optical excitation to transition the contrast agent to a triplet state, with associated absorption changes. But it has limited application.
Molecular contrast can also be generated by nonlinear optical interactions that generate photons coherent with the excitation field. Both second harmonic generation (SHG) and coherent anti-Stokes Raman (CARS) have been examined as molecular imaging schemes for OCT. SHG has declined in popularity as the combination of OCT and multiphoton microscopy has gained attention, while the complexity of CARS has limited its development and use.
The use of scattering contrast agents is also promising for OCT. For example, plasmonic nanoparticles can be used in place of fluorescent markers, and gold nanoparticles (for absorption, photothermal excitation, or both) have potential, though gold's molecular specificity has not been demonstrated.
While the combination of OCT and molecular imaging is promising for correlating imaging to disease physiology, it has been limited to animal studies, and work is needed for clinical translation. Potential applications include detection of neoplastic processes and arterial oxygenation. Further developments of PPOCT indicate potential for diagnosis of ocular melanoma. While fluorescence-guided OCT offers diagnostic potential, it does not extend OCT's depth-gating capabilities to molecular specificity. Cardiovascular application has been explored using a probe based on a double-clad fiber (DCF) combiner.
Serving a critical need
Medical diagnostics currently relies on histopathology to analyze the micro-scale structure of tissues as a complement to macro-scale imaging techniques such as ultrasound and computed tomography. By collecting high-resolution information in vivo and noninvasively, OCT provides unprecedented capabilities. By extending OCT to provide functional information, a critical clinical need to increase specificity can be realized, enabling more diagnostics and extending the utility of this powerful imaging technique.
REFERENCES
1. J. Kim, W. Brown, J. R. Maher, H. Levinson, and A. Wax, Phys. Med. Biol., 60, 10, R211-R237 (2015).
2. H. C. Hendargo et al., Biomed. Opt. Express, 4, 6, 803-821 (2013).
3. E. Götzinger, M. Pircher, and C. K. Hitzenberger, Opt. Express, 13, 10217-10229 (2005).
4. S. G. Adie et al., Opt. Express, 18, 25, 25519-25534 (2010).
Adam Wax is a professor of biomedical engineering at Duke University, Durham, NC; e-mail: [email protected]; http://bios.bme.duke.edu.