JONATHAN HU and CLAIRE GMACHL
Trace-gas analysis and chemical sensing using quantum cascade lasers (QCLs) has attracted more attention in recent years since most molecules have a unique absorption spectrum in the mid-infrared (mid-IR) region. With many commercial options available, QCLs are ideal candidates for mid-IR spectroscopy because their emission wavelength reaches from the mid-IR to the far-IR with high power efficiency.1
The conventional semiconductor laser is based on electrons transitioning between the conduction band and valence band, with photon emission taking place as a result of the recombination of carriers across the bandgap. These QCLs are based on the intersubband transitions between quantized subbands in one band—usually the conduction band—in multiple-quantum-well heterostructures, which is fundamentally different from conventional semiconductor lasers.
Since QCLs use intersubband optical transitions, they were once thought to be inefficient and their emission spectra were considered to be inherently limited in tunability and breadth. However, recent research shows that different quantum designs of the heterostructure can be implemented to significantly improve the performance of QCLs.2 A widely voltage-tunable intersubband emission can be achieved via Stark effect in coupled quantum wells, and transitions from the multiple subbands yield a broadband spectrum.3,4
The National Science Foundation’s Engineering Research Center on Mid-InfraRed Technologies for Health and the Environment (MIRTHE; www.mirthecenter.org; Princeton, NJ) develops QCL-based mid-IR chemical sensors for environmental monitoring, homeland security, and medical diagnostics. In this article, we will present selected examples of recent developments for quartz-enhanced photoacoustic spectroscopy, Faraday rotation spectroscopy, a QCL open-path system, and a wireless sensor network over Princeton.
Quartz-enhanced photoacoustic spectroscopy
Acoustic waves in gas samples can be produced by the absorption of acoustically modulated laser radiation at acoustic frequencies by the target trace-gas species. Such photoacoustic spectroscopy is an indirect technique in which the effect on the absorbing medium is detected instead of direct light attenuation. Only absorbed light contributes to the photoacoustic spectroscopy signal, and drawbacks of regular spectroscopy with background absorption and scattered light do not play a role.
A novel and recently much improved approach to using quartz-enhanced photoacoustic spectroscopy (QEPAS) is to accumulate the acoustic energy in a sharply resonant acoustic transducer in the form of a quartz tuning fork. This QEPAS technique has been applied to the detection of various chemical species, such as ammonia (NH3), water (H2O), carbon dioxide and carbon monoxide (CO2 and CO), nitrous oxide (N2O), and formaldehyde (CH2O).5
In one QEPAS example, a rapidly tunable external-cavity QCL from Daylight Solutions (San Diego, CA) was used as an excitation source to detect and quantify molecules with wide and quasi-unstructured absorption bands. The QCL is continuously tunable in the 1196–1281 cm−1 spectral range, which matches the absorption spectra of the studied molecules.6
The QEPAS technique has many unique properties such as an extremely high quality factor of more than 10,000; rapid spectral measurement; immunity to environmental acoustic noise; high sensitivity; and wide dynamic range. When the microresonator tubes are cut to the correct length to match the resonant frequency of the quartz tuning fork, the sensitivity of QEPAS can be increased 1.5–2 times. Measurement precision of 13 parts per billion by volume (ppbv) for pentafluoroethane and 15 ppbv for nitrogen oxide concentrations can be achieved.7,8
Faraday rotation spectroscopy
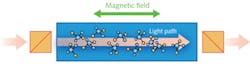
Faraday rotation spectroscopy (FRS) exploits the magnetic circular birefringence (MCB) observed in the vicinity of Zeeman split absorption lines and provides enhanced detection of paramagnetic molecules such as nitric oxide (NO), nitrogen dioxide (NO2), hydroxide ions (OH-), or oxygen (O2).9 The paramagnetic molecules exhibit MCB when an external magnetic field is applied in the same direction as the light propagation. The magnetic field along the direction of optical beam propagation induces the circular birefringence. The refractive index is different for left-handed and right-handed circularly polarized waves, which leads to rotation of the polarization axis of linearly polarized light (see Fig. 1).
Using a broadly tunable external-cavity QCL allows the selection of the optimum line for the FRS detection of nitrogen oxide and provides flexibility in selecting the required laser wavelength to perform sensitive detection of paramagnetic species. Precisions of 4.3 ppbv and 0.38 ppbv to detect atmospheric nitric oxide using only 44 cm effective optical path length are obtained by using a thermoelectrically cooled mercury cadmium telluride photodetector and liquid-nitrogen-cooled indium antimonide photodetector, respectively.10
QCL open-path system
The MIRTHE QCL open-path system (QCLOPS) was designed to use laser absorption spectroscopy for detecting a range of trace gases in field deployment with a broadly tunable QCL from Daylight Solutions as the light source. Open-path sensors are important, for example, in air quality measurements.
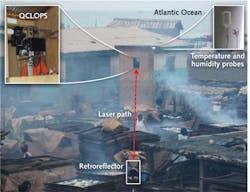
The QCLOPS system can potentially monitor ozone, ammonia, and carbon dioxide, and was recently tested in sensing complex chemicals in wood smoke in the rural fishing village of Elmina, Ghana in the summer of 2010 (see Fig. 2).11 Two gases from the wood smoke, 2-methyl phenol and benzyl alcohol, were targeted and successfully identified by QCLOPS. These gases were chosen because they are harmful and have fingerprints in the emission region of the available QCL (965–1260 cm−1).Wireless sensor network over Princeton
To make a testbed for the development of new sensor technologies, a wireless sensor network over Princeton (SNOP) has been installed. The SNOP includes 12 wireless stations deployed over the Princeton University campus at ground and roof levels (see Fig. 3). The sensor instruments include an IR thermometer for measuring surface temperature, a solar radiation sensor, a three-dimensional sonic anemometer, an open-path IR gas analyzer, a temperature and relative humidity probe, an IR surface temperature sensor, and a four-component radiometer.12The data collected from SNOP can be visualized and downloaded in quasi-real time at http://snop.princeton.edu. The SNOP wireless network will help MIRTHE and its academic and industrial collaborators develop next-generation mid-IR sensing technology that will hopefully lead to commercial sensing product lines important to numerous industries worldwide.
ACKNOWLEDGMENTS
We would like to acknowledge the support from NSF-ERC (Grant No. EEC-0540832). SNOP is supported previously by the High Meadows Sustainability Fund of Princeton University. This work highlights recent achievements by individuals and groups: specifically C. Amuah, E.N. Bentil, E. Bou-Zeid, P. Buerki, R. Curl, T. Day, L. Dong, J. Doty, M. Eghan, C. Gmachl, E. Jeng, A. Kosterev, R. Lewicki, A. Malinovsky, A. Michel, I. Morozov, M. Reed, D. Serebryakov, J. Smith, S. So, V. Spagnolo, D. Thomazy, F. Tittel, Z. Wang, and G. Wysocki.
REFERENCES
1. R.F. Curl et al., Chem. Phys. Lett., 487, 1–18 (2010).
2. Y. Bai et al., Nature Photon., 4, 99–102 (2010).
3. Y. Yao et al., Appl. Phys. Lett., 95, 021105 (2009).
4. Y. Yao et al., Appl. Phys. Lett., 97, 081115 (2010).
5. A.A. Kosterev et al., Rev. Sci. Instrum., 76, 043105 (2005).
6. L. Dong et al., Appl. Phys. B, 100, 627–635 (2010).
7. A. Kosterev et al., Appl. Phys. B, 100, 173–180 (2010).
8. V. Spagnolo et al., Appl. Phys. B, 100, 125–130 (2010).
9. S. So et al., Appl. Phys. B, 102, 279–291 (2011).
10. R. Lewicki et al., Proc. Natl. Acad. Sci., 106, 31, 12587–12592 (2009).
11. E.N. Bentil et al., CLEO 2011, Baltimore, MD, paper JMC6 (2011).
12. Z. Wang et al., Boundary-Layer Meteorology, 138, 2, 171–193 (2011).
Jonathan Hu is an assistant professor in the Department of Electrical and Computer Engineering at Baylor University, Rogers 301D, One Bear Pl. #97356, Waco, TX 76798; e-mail: [email protected]; www.baylor.edu. Claire Gmachl is MIRTHE director and Eugene Higgins Professor of Electrical Engineering at Princeton University, B326 Engineering Quadrangle, Olden St., Princeton, NJ 08544; www.princeton.edu.