Microscopes have given scientists vital insight into living things ever since Robert Hooke and Antoni van Leeuwenhoek discovered the microscopic world in the 17th century. Now the quest for better views of living cells is pushing development of new types of optical microscopy that overcome the traditional limitations without sacrificing the advantages of visible light.
The resolution of conventional optical microscopy is inherently limited to roughly half the wavelength and suffers from limited contrast in brightness and refractive index between cell components, making it hard to resolve fine details. But visible wavelengths also have important advantages. They can penetrate cells and tissue, allowing examination of internal structures. Unlike shorter-wavelength light or electron microscopy, optical microscopy doesn’t kill cells or require pretreatment that kills them, allowing in vivo studies. Thanks to these advantages, roughly 80% of life-science microscopy is done with conventional optics and lenses.
Considerable improvements have occurred in recent years, including new techniques that improve far-field resolution beyond the classical half-wavelength limit, enhance the effective contrast to pick out particular elements of cells, and improve speed and sensitivity. Confocal microscopy and optical coherence tomography (OCT) have already reached clinical use. Other new techniques take advantage of optical nonlinearities, fluorescence effects, or phase-sensitive techniques.
Confocal microscopy and OCT
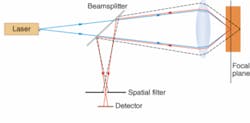
Many new techniques draw on confocal microscopy, in which an object is scanned with a bright spot and a spatial filter in front of the detector records only light returned from the spot being illuminated. The confocal method is particularly valuable in fluorescence microscopy—in which the illuminating beam excites fluorescence from parts of the sample—because it suppresses background noise from areas outside the focal point, both in the same plane and in other layers (see Fig. 1). Images are built up by raster scanning, and in transparent materials slices can be taken at different depths.
Optical coherence tomography is an interferometric technique that uses low-coherence light to examine tissue at depths of a millimeter or two (see www.laserfocusworld.com/articles/201664). Input light is divided between two arms of an interferometer, one serving as the reference beam, and the other delivering light to the tissue being studied. Combining light reflected from the sample with the scanned reference beam reveals reflective structures within the tissue. Scanning in three dimensions by moving the optics or sample builds up an image. Penetration depth is limited by light scattering, but OCT has been adopted in fields such as ophthalmology because it can provide resolution finer than a micrometer—much higher than acoustic imaging—and does not expose patients to ionizing radiation.
Fluorescence microscopy
A well-established technique, fluorescence microscopy is based on adding dyes that selectively bond to target structures within cells, and that fluoresce when illuminated by a suitable light source. Filtering out the excitation wavelength leaves an image showing distribution of the fluorescent dye, revealing the compound or structure it bonds to, such as DNA.
However, single-photon excitation requires a short-wavelength source, which can “cook” cells and does not provide depth resolution. Development of femtosecond near-infrared lasers led to the demonstration of two-photon scanning fluorescence microscopy by Winifried Denk and Walt Webb at Cornell University (Ithaca, NY).1 Tightly focusing ultrashort infrared pulses produced the high intensities needed to excite two-photon fluorescence on only a tiny spot—avoiding flash-frying the sample and providing depth resolution. The infrared photons also could penetrate farther into cells and tissue. “Two-photon fluorescence is the gold standard” for research, says Jeff Squier of the Colorado School of Mines (Golden, CO), allowing biologists “to visualize functionality that they had never been able to see before.”
Squier has used two-photon fluorescence microscopy to simultaneously image two focal planes by toggling focus back and forth between them.2 Another example of the power of the technique is an experiment in which David Kleinfeld of the University of California at San Diego (La Jolla, CA) monitored blood from the brains of animals in three dimensions, after he induced aneurisms, to help understand the impact of strokes.3
By developing sophisticated optical systems, Stefan Hell of the Max Planck Institute for Biophysical Chemistry (Göttingen, Germany) has achieved 80 to 150 nm resolution in three-dimensional cell images. Adding a technique called stimulated emission depletion, based on saturating a fluorescent transition in selected areas, improved resolution to 33 to 60 nm.4
Fluorescence microscopy can be extraordinarily sensitive. Sunney Xie at Harvard University (Cambridge, MA) has detected single fluorescent protein molecules by getting them to stay localized at one point in a living bacterium. Normally, fluorescent molecules in cytoplasm move constantly at speeds fast enough to take them across the entire cell in about ten milliseconds. Xie’s trick is to bind the fluorescent protein to DNA, which stays essentially fixed during imaging. The method has been used to study protein interactions with DNA in living cells.5
Nonlinear microscopy
The clinical use of fluorescent dyes is limited by their tendency to be carcinogenic or toxic. “If you’re going to do something in humans, you can’t be adding fluorescent dyes,” says Paul Campagnola at the University of Connecticut Health Center (UConn; Farmington, CT). To avoid fluorescent dyes, he and others have turned to nonlinear techniques that can make intercellular structures or tissues emit light directly.
Campagnola and William Mohler are developing an approach at UConn that involves generating second-harmonic radiation in proteins already present in cells by illuminating them with ultrashort near-infrared pulses with high peak power. “The contrast agents are already there, the proteins in the animal or the patient tissue that glow if you excite them with the right laser. And they are very stable,” unlike fluorescent dyes, says Mohler. He’s particularly impressed by direct measurements in living mice of the length of sarcomeres, a basic unit of muscle that had never been seen in vivo before.6
Campagnola is focusing on ovarian cancer, which changes the arrangement of collagen in soft tissue called stroma found in the ovary. “We can see those changes,” Campagnola says, because they affect the nonlinear properties of the collagen. Ovarian cancer is among the family of epithelial cancers—including breast, prostate, pancreatic, and skin cancers—that account for about 85% of all cancer, and he thinks similar changes should be observable in those types. Collagen also changes in most muscular and skeletal diseases. He says the second-harmonic technique could be used endoscopically, with a fiber-optic probe delivering laser light to the ovary.Third-harmonic generation from endogenous material is another possibility. Because third-harmonic generation relies on different physics, it is attractive for applications in which there are changes in refractive index. It could be used to spot fat bodies in cells, or fatty deposits in muscles, which have a high index contrast with the surrounding material.7
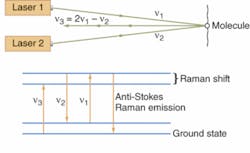
Another nonlinear approach uses CARS (coherent anti-Stokes Raman spectroscopy), a four-photon process (see Fig. 2). The lasers are tuned so their frequency difference matches the Raman shift of small molecules found in the cell. Coherent interactions with the molecules produce four-frequency mixing, with two photons at one frequency ν combining with one photon at the Stokes frequency ν2 (the first laser frequency minus the Raman shift) to produce a photo at the anti-Stokes frequency ν3 (the first laser frequency plus the Raman shift): 2ν1 - ν2 = ν3. Concentrating the power of the two laser beams on a small point generates a strong nonlinear effect, which generates anti-Stokes emission from small molecules present in quantities of thousands to millions. That’s important because small molecules such as drugs are otherwise hard to detect, and adding fluorescent labels would change their properties.
Xie reports dramatic increases in sensitivity since his team’s first experiment in 1999, so images can be recorded at video frame rates. Magnetic resonance imaging penetrates more deeply, but its low sensitivity requires long data collection times and its spatial resolution can’t match the 300 nm of CARS. Stretching vibrations of C-H bonds produce strong CARS signals; applications include studying lipid metabolism and real-time imaging of brain tissue.8, 9 Drug companies are excited because by replacing hydrogen atoms with deuterium, they can shift the Raman emission to a lower frequency where there are no other important peaks, making it possible to map drug distribution in cells.Phase microscopy
Other new techniques are aimed more at research than clinical needs. One particularly hot topic is phase microscopy, says Adam Wax of Duke University (Durham, NC). It records phase-contrast images by quantitatively measuring phase changes using interferometry or other techniques.
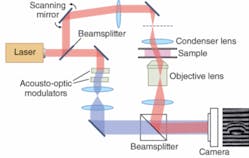
Last year Wonshik Choi and Michael Feld at MIT (Cambridge, MA) reported three-dimensional mapping of refractive-index variations in living cells and tissue using a phase-shifting interferometer that varied illumination angle to scan the cells (see Fig. 3).10 Wax has shown that asynchronous digital holography can measure phase quantitatively.11 Spectral-domain phase microscopy, developed by Wax’s Duke colleague Joe Izatt, can achieve high resolution in depth.12
Outlook
The current microscopy boom owes much to the National Institutes of Health (www.nih.gov), which has drawn optics specialists into biomedical applications by generous funding to develop new and better clinical diagnostics as well as improved research tools. The resulting torrent of new papers can be overwhelming.
No single technique is likely to dominate because none can do everything well. There is a need to strike a balance among goals such as high resolution, fast imaging, strong contrast, and high sensitivity. Different approaches will be needed in the clinic and the laboratory, or for examining different structures. Campagnola says the best approaches are likely to come from combining techniques, so the future is going to be multimodal.
REFERENCES
1. W. Denk, J. Strickler, W. Webb Science 248(4951) 73 (1990).
2. W. Amir et al., Optics Lett. 32, 1731 (June 15, 2007).
3. N. Nishimura, et al., Proc. National Academy of Sciences 104, 365 (January 2, 2007).
4. S.W. Hell, Science 316, 1153 (25 May 2007).
5. J. Elf et al., Science 316, 1191 (2007).
6. M. Llewellyn et al., paper at 2007 annual meeting of American Society of Biomechanics.
7. Y. Barad, H. Eisenberg, M. Horowitz, Y. Silberberg, Appl. Phys. Lett. 70, 922 (1997).
8. X. Sunney Xie et al., Science 312, 228 (April 14, 2006).
9. Evans et al., Optics Exp. 15, 12076 (2007).
10. W. Choi et al., Nature Methods 4, 717 (2007).
11. K. Chalut et al., Optics Exp. 15, 3047 (March 19 2007).
12. M.A. Choma, et al., Optics Lett. 30, 1162, (May 15, 2005).