Researchers at the Massachusetts Institute of Technology (MIT; Cambridge, MA) are patenting a microscope-compatible device that combines microfluidics and laser optics in a potentially simple, effective and low-cost alternative for bioscience applications, such as cell sorting.
Cell sorting is an old and ubiquitous problem, according to Joel Voldman who heads the MIT research team, whether isolating one cell in a thousand or looking for a certain subpopulation that behaves differently. “Often we want to do something with the cells, in some cases simple or in others complex, like creating genetic differences, perhaps associated with a disease,” Voldman said. “The trick is in figuring out how to get the ones you want, particularly when they are very small. A microscope can be used to see them, but how does one go about manipulating them?”
The primary traditional methods of cell manipulation are fluorescence-activated cell sorting (FACS), which enables high-throughput sorting based on fluorescent probes (rather than visual inspection), and laser capture microdissection (LCM), which allows a user to base selection on visual (but not real-time) images. Commercially available LCM systems have been adapted specifically for cell sorting, but tend not to be used widely due to relatively high cost.
“Even though there are probably thousands of microscopes in individual labs at MIT, there are only a handful of LCM systems,” Voldman said. “So the tack we followed, which is the hallmark of our lab, was to ask, ‘What’s the simplest way to make it work?’
In answering that question, Voldman and graduate student Joseph Kovac took a cue from previous work in which laser tweezer techniques were used to either tweeze or push targeted cells to desired locations. The high numerical-aperture (NA) requirements of laser tweezers impose field size constraints that make it difficult to work with mammalian cells, however. Such systems also have relatively high optical power requirements. Optoelectronic tweezers (OETs) based on lower-NA optics had been tried also, but pose similar performance compromises.
Microfluidics and lasers
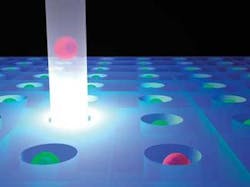
Ultimately, the researchers chose to combine two technologies: microfluidics for introducing, inspecting, and removing cells from the system; and laser optics for selecting and manipulating individual cells.1 Their microfluidic structure consisted of a two-dimensional array of single-cell-size wells fabricated into a chip that they attached to the underside of a microscope slide. Cells were introduced and removed from the chip via fluid flow, and cells that descended wells were protected from fluid flow and thereby retained on the chip.
A simple laser system was incorporated into the microscope system to levitate cells of interest out of the microwells and into the fluid flow. Voldman likened the levitation process to pushing a beach ball with the water jet from a fire hose (see figure).
“We are essentially using the concept of optical tweezers, which are enabled by a fairly high-powered laser and a fairly high-numerical-aperture optic. If you do this so that it doesn’t work, with a low-powered laser and a low-NA optic, all that happens is that you propel the object along the laser axis. Pushing one cell upward does not take much force, only about a piconewton. So we basically created an optical tweezer that doesn’t tweeze, but that does what we want.”
In addition, the low-divergence beams, relatively large spot sizes, and low peak intensities actually made Voldman’s system more effective and compatible for work with mammalian cells than systems based on working optical tweezers.
The laser source in the MIT device was a relatively inexpensive, computer-controlled 980 nm fiber-coupled semiconductor diode laser, capable of outputting up to 290 mW of single-mode output power, about on-third of which was actually delivered to targeted cells. The researchers coupled the laser beam into the microscope using a cage-mounted collimation/focusing apparatus. Voldman and Kovac wrote software to control the process.
Using the device in conjunction with a vertical microscope, the researchers successfully demonstrated image-based cell sorting on whole-cell fluorescence and on localized fluorescence within a cell. They believe that the simple microfluidic and optical sorting architecture can be scaled upward to handle more than 10,000 individually addressable wells. The next steps in developing the technique involve engineering improvements to make the system more efficient, sterile and so on. And ultimately, going to market will require the acquisition of a commercial partner.
Voldman emphasized, however, that the new device will play a complementary rather than competitive role with existing technology. “For throughput, flow sorting (FACS) is the way to go. It can give you 104 cells per second. And LCM was not developed for live cell sorting, but for tissue sampling, as in a biopsy, where you cut out a tumor then take a sample of it,” he said. “This method works better than the others when cells are alive and the parameter you are sorting on is something you can see with your eyes.”
REFERENCE
1. J.R. Korvac, J. Voldman, Analytical Chem. 79(24) 9321 (Dec. 15, 2007).