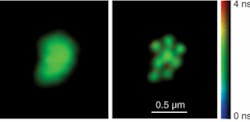
Stimulated emission-depletion microscopy represents one of the most straightforward among techniques to image at a resolution beyond the diffraction limit. Now, it has been combined with fluorescence lifetime imaging (FLIM) to create a truly novel microscopy with sub-diffraction-limit resolution in three dimensions.
Stimulated emission-depletion microscopy (STED) works by selectively suppressing the fluorescence at the periphery of an excited region to be imaged. An excitation pulse irradiates the region first, and a synchronized, red-shifted second pulse—the STED pulse—acts only on the excited molecules to induce stimulated emission. The STED beam is made to be doughnut-shaped, so that the depletion region is an annulus around the central imaging region. This saturation of the fluorescence quenching is key; by increasing the intensity of the STED beam—which maintains its zero intensity at the center—the still-fluorescing region to be imaged can be narrowed further and further, in principle to any size.
Egidijus Auksorius and colleagues at Imperial College London have now made the idea more flexible by providing a tunable, broadband source for the excitation beam. They focus 100-fs-long pulses from a Ti:sapphire laser into a 1-m-long microstructured optical fiber (MOF), leading to a supercontinuum that spans from 515 nm to more than 1 µm. A portion of the Ti:sapphire beam is then stretched to about 300 ps and used as the STED beam.
The crucial step, though, is the implementation of a programmable, liquid-crystal-based spatial light modulator (SLM) to optimize the STED beam. Placing the modulator in a plane conjugate to the microscope objective allows a holographic phase-modification approach for the STED beam. Digitally generated “blazed holograms” from the SLM imprint the desired phase distributions on the first-order diffracted STED beam. A helical phase ramp is optimal for lateral resolution, whereas a centrally located disk with a half-wave phase shift is ideal for axial resolution. Further modifications to the holograms also correct for aberrations in the depletion region. The beams are scanned across a sample using galvanometers to acquire an image on a PMT, and by parsing the signal from the PMT in time FLIM images can be built up.1
This microscopy technique holds much promise for biological imaging, as it represents the first demonstration of FLIM imaging with subdiffraction far-field resolution. “This, we expect, could be useful to study protein interactions using FLIM-FRET [fluorescence resonance energy transfer], to study nanoclusters of cell signaling, or to study subresolution features such as ‘lipid rafts’ with the better resolution,” lead author Auksorius says.
Microscope modification
What’s more, the team implemented the approach in a commercial confocal microscope, a good sign that the approach can be an “add-on” to existing bioimaging setups. “STED is a sort of natural extension to many of the established contrast modes used in laser-scanning confocal fluorescence microscopy,” says Stefan Hell, director of the department of nanobiophotonics at the Max Planck Institute for Biophysical Chemistry (Göttingen, Germany). Hell invented the method in 1994 and demonstrated the first STED microscope in 1999.2 “The reason this paper is important is that they do it in a standard beam-scanning commercial confocal laser-scanning microscope, thus showing that current confocal laser-scanning microscopes equipped with FLIM can be modified to become subdiffraction-resolution FLIM microscopes.”
The researchers then went on to show that a single source could provide both beams, with added advantages that will make the new microscopy widely applicable. “We plan to employ fiber-laser-based supercontinuum sources because such sources are commercially available, compact, and powerful, and can access almost all wavelengths necessary for both excitation and depletion of the dyes most commonly used in biological research,” says Auksorius. “They are also cheap compared to Ti:sapphire lasers that are routinely used for nonlinear microscopy and FLIM.”
The stability of the galvanometric scanners is the limiting factor in the ultimate resolution of the setup, but work much more quickly than the more stable piezo scanners. That, says Auksorius, is a necessary feature as the group moves on to live-cell studies with the new approach.
REFERENCES
1. Auksorius et. al., Opt. Lett. 33, 113 (2008).
2. T.A. Klar and S.W. Hell, Opt. Lett. 24, 954 (1999).