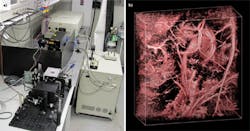
A nonionizing laser shoots through the skin into tissue and cells beneath the surface. The absorbed laser energy turns into heat, which sets off an ultrasonic emission that can be captured and converted into images. This technology, photoacoustic imaging, promises better views of mechanisms in organs, cells, subcellular structures, and even biochemicals. To make this technique widely available, though, researchers need commercial instruments, which are on the way.
To see life in its natural state, researchers want-as much as possible-imaging approaches that need no stains or labels. Photoacoustics provide this with "rich contrast," according to Lihong Wang, Gene K. Beare Distinguished Professor at Washington University in St. Louis, and inventor of three-dimensional photoacoustic microscopy (see http://bit.ly/h2EUI5). The key is the wide range of biological materials that absorb the laser energy. "Almost anything absorbs it," he says. The list includes oxyhemoglobin, deoxyhemoglobin, melanin, lipids, DNA, RNA and more. So, as Wang says, "Photoacoustics can image cell nuclei in vivo without staining."
The same cannot be said for other popular imaging techniques. For example, many molecules do not fluoresce, so they must be labeled to see them with fluorescent microscopy. "Then, we have to ask if the dye is safe in vivo," Wang points out. With photoacoustic microscopy and no labels, researchers can view anatomical structure and function.
Moving into microscopy
Photoacoustic microscopy started as a two-dimensional approach used in industry. "People used it to image the surface of materials-nonbiological materials, like metals," says Wang. "We added time of arrival of the acoustic waves to get depth resolution. This turned 2D into 3D."
Now, Wang and his team push the third dimension of photoacoustic microscopy even deeper into tissue. To get more depth, though, spatial resolution decreases, and vice versa. "We've extended the imaging depth from 1 mm to 7 cm," he says. "So photoacoustic microscopy extends the depth of standard optical microscopy and has broken through what is called the optical diffusion limit for all existing optical microscopy technologies." So far, 220 nm is the highest resolution that Wang has reached with superficial optical-resolution photoacoustic microscopy.
To make this technology available to more researchers, Wang is working with Microphotoacoustics in New York, which has licensed his patents in hopes of making a commercial photoacoustic microscope. He also mentions working with Olympus on a multimodality microscope that would include photoacoustics along with confocal and two-photon microscopy.
As Wang says, "We provide a tool. We're willing to be the guys who serve the biomedical research and clinical communities."
Setting up a system
So far, researchers wanting to do biological research using photoacoustics have had to build their own systems. Increasingly, the pieces necessary for construction of such systems are becoming available for purchase. For example, GWU (Erftstadt, Germany) makes tunable light sources useful for photoacoustics. According to Günter Warmbier of GWU, the company's "most popular product is an OPO [optical parametric oscillator] pumped by a frequency-doubled Nd:YAG laser, which will be formally released soon." He adds, "We have several pre-production units in the field in customer labs."A tunable laser lets users find the wavelength that works best for the material at hand. Different tissues and molecules absorb some laser energy better than others.
Nonetheless, getting a homemade photoacoustic system up and running takes some expertise. According to Roger J. Zemp, assistant professor of electrical and computer engineering at the University of Alberta and maker of his own systems, "It helps to have some experience in the field. It can be fairly tricky." The key issues revolve around setting up the parameters of the laser, focusing the light, receiving the acoustic signals and developing integrated scanning hardware and software.
For those with the expertise to make their own system, the price is dropping. Zemp mentions using a laser system in the past that cost $150,000, but says that a microchip laser can now be used for optical-resolution photoacoustic microscopy and purchased for less than $10,000. He adds, "A fiber laser can do a bit more, but it's slightly more expensive." Eventually, he expects to see a shoebox-size instrument.
Adding applications
VisualSonics (Toronto, ON, Canada) got into the high-frequency ultrasound market more than a decade ago. In recent years, this company wanted to expand the applications of its Vevo systems. "We want to provide applications that answer the biological questions that researchers deal with on a regular basis," says Catherine Theodoropoulos, director, product management at VisualSonics, "and we want to do that in vivo and in real time with extremely high resolution and sensitivity." Those desires focused VisualSonics on photoacoustics.
Early in 2011, VisualSonics introduced the Vevo LAZR photoacoustic imaging system, which is its Vevo 2100 system plus a tunable laser (670–980 nm), which adds the photoacoustic capability. Researchers who already own a Vevo 2100 system can upgrade to the photoacoustic version with the integration of the laser unit. "The photoacoustic side gives researchers a tool to look at nanoparticles and agents in vivo and real time, and understand what they are doing," Theodoropoulos says.
For now, VisualSonics aims the Vevo LAZR platform primarily at cancer biology. For one thing, this instrument can be used to study angiogenesis and blood flow related to tumors by quantifying oxygen saturation.
Like the Vevo 2100 system, the Vevo LAZR allows imaging of many organs, but with some constraints. With this photoacoustic system, researchers can image at high-resolution to about one centimeter. "We will improve on that," Theodoropoulos says.
Beyond cancer research, the Vevo LAZR technology could also be used in hemodynamics, or movement and functional status of the blood. Theodoropoulos adds that they "are getting preliminary data in brain, embryos and muscle."
Imaging dynamic processes
Since photoacoustic approaches can deliver 2D or even 3D images in real time, they might be applied to some of the deepest mysteries in biology. That's exactly the goal of Daniel Razansky, head of the Laboratory for Experimental Biological Imaging Systems and deputy director of the Institute for Biological and Medical Imaging at the Technical University of Munich and Helmholtz Center Munich in Germany. He is working on photoacoustic systems that will image dynamic processes, such as deep brain activity or real-time uptake of molecular agents.
He says, "My lab is designing and building this system all the way from instrument development, signal processing, and algorithmic research to validation in small animal studies and, potentially, clinical trials."
Razansky uses multispectral optoacoustic tomography (MSOT) to add molecular contrast materials—like dyes, proteins, and nanoparticles—in order to expand to even more molecular and functional imaging applications. "Maybe," he says, "we'll be able to image early molecular indications related to diseases, such as cancer, atherosclerosis, and Alzheimer's, or monitor efficacy of therapies and drugs in living tissues."
As Razansky's team expands its uses of photoacoustics, he also helps with the creation of commercial products. In 2010, one such product—the MSOT PCS-2 small animal scanner—was commercially launched by Munich-based iThera Medical.
As the basic research continues and more commercial products become available, we will be amazed by what we will soon see, thanks to laser-induced sound.
Editor's Note: This article first appeared in the March/April 2011 issue of BioOptics World.
![FIGURE 1. Photoacoustic microscopy provides anatomical, chemical, and dynamic imaging. Using 584 nm light to image a mouse ear bearing a xenotransplanted B16 melanoma tumor (white-dashed box in [a]), this image reveals a principal artery-vein pair (magenta-dashed box in [a]) that feeds and drains the tumor region. In this image, depth is color-coded: blue (superficial) to red (deep). For comparison to traditional microscopy, see the white-light photograph of the mouse ear (b), which is shown at lower resolution. Photoacoustic technology can also reveal melanin (c), and here the blood vessels are invisible due to the weak absorption of hemoglobin at the wavelength used. For examples of dynamic imaging, see the photoacoustic images of oxygen saturation in the principal arterial-vein pair revealed with dual-wavelengths excitation at 584 and 594 nm (d) and flow velocity imaging of the principal arterial-vein pair (e). The arrows show the directions of positive and negative flow, and pulsing is even visible. FIGURE 1. Photoacoustic microscopy provides anatomical, chemical, and dynamic imaging. Using 584 nm light to image a mouse ear bearing a xenotransplanted B16 melanoma tumor (white-dashed box in [a]), this image reveals a principal artery-vein pair (magenta-dashed box in [a]) that feeds and drains the tumor region. In this image, depth is color-coded: blue (superficial) to red (deep). For comparison to traditional microscopy, see the white-light photograph of the mouse ear (b), which is shown at lower resolution. Photoacoustic technology can also reveal melanin (c), and here the blood vessels are invisible due to the weak absorption of hemoglobin at the wavelength used. For examples of dynamic imaging, see the photoacoustic images of oxygen saturation in the principal arterial-vein pair revealed with dual-wavelengths excitation at 584 and 594 nm (d) and flow velocity imaging of the principal arterial-vein pair (e). The arrows show the directions of positive and negative flow, and pulsing is even visible.](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/01/1203lfw_fea1_fig1.png?auto=format%2Ccompress&w=250&width=250)