The US Naval Research Laboratory (NRL; Washington, DC) is aiming to sort particles in biological fluids using laser-based, optofluidic methods that do not require prior knowledge of DNA sequence attributes or attachment of antibodies and/or fluorescent tags.1 The NRL research instead uses optical forces that arise when laser light interacts with microscopic particles. Forces vary due to inherent differences in optical pressure or force due to a particle’s size, shape, refractive index, or morphology to perform particle separation. The label-free optical chromatography system, which uses a microfluidic chip and the flow of a fluid in the opposite direction of a laser beam, can successfully identify different blood cell types and determine their sorting parameters.
Optical chromatography
Unlike flow cytometry, optical chromatography based on optical force separation of particle types does not require a fluorescent or antibody tag, but relies on the balance of optical and fluidic forces on a particle in a microchannel. The concept of optical force, in which a laser or light beam exerts a fraction of its momentum on a particle through scattering, refraction, or other particle interactions, is well known in the biomedical community through techniques such as optical tweezing/trapping and optical lift.
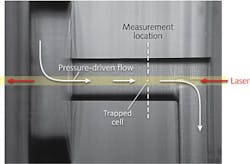
The flow cell consists of three square plates of fused silica, each with a side length of 25.4 mm and a thickness of 1 mm. The center plate has a structure 250 μm wide × 200 μm deep on both sides of the plate, connected by two 250-μm-diameter capillary through-holes. These through-hole channels are the regions in which particles and cells are trapped and analyzed. A 1-mm-diameter, 200-μm-thick circular plate at the channel entrance focuses the fluid flow, and three 350-μm ports are drilled into the flow cell for inlet, outlet, and sample-injection locations.
A 3 W continuous-wave 1064 nm fiber laser is focused by a 0.5-in.-diameter, 100-mm-focal-length lens in the opposite direction of the fluid flow. The flow cell is mounted on a five-axis positioner, lit with a commercial fiber-optic illuminator, and magnified with a 10X objective before being imaged with a CCD camera.
To understand the flow and particle-separation dynamics of different blood-cell types, purified fractions of lymphocytes, monocytes, granulocytes, and erythrocytes diluted in solution to a concentration of 100,000 cells/mL were flowed into the microfluidic system (see figure).
Sorting parameters
As individual cells become trapped within the beam path, the fluid-flow rate of the system is adjusted so that the cells come to rest. And because the fluidic and optical forces are equal at this point, the trapping flow rate for different particles can be measured. For the blood-cell components analyzed and plotted in a histogram, trapping flow rates were as low as 196 ± 34 nL/min for nominal 8.4-μm-diameter lymphocytes and 754 ± 65 nL/min for nominal 12.2-μm-diameter erythrocytes; however, flow rate did not vary according to cell size alone; refractive index and other morphological parameters of the cell are responsible for the flow-rate differences.
The next step in the NRL research is to use the blood-cell sorting parameters to design a fully functional optofluidic system that could automate the separation of the various blood-cell types based on their different trapping flow rates. “The subtleties of optical force differentials among biological cell types are very fine and this represents a tremendous opportunity to harness a label-free system for sample characterization and sorting,” says Sean J. Hart, research chemist at NRL. “Removing the requirements of prior knowledge [antibodies or DNA primers] will allow more rapid and cost-effective systems to be developed. Furthermore, an instrument that can identify a new and perhaps yet unknown cell type and sort it from related populations will greatly benefit scientific discovery in a way that current systems cannot.”
REFERENCE
1. S.J. Hart, OSA Frontiers in Optics conference paper FMA1, San Jose, CA (Oct. 17, 2011).