TUNABLE SOURCES: Quantum cascade lasers enter industrial applications
BOB SHINE, PETER R. BUERKI, and TIM DAY
The mid-infrared (IR) portion of the electromagnetic spectrum (~3–20 µm) is an extremely fertile spectroscopic region. Quantum cascade lasers (QCLs), first introduced in 1994, have expanded access to this region by offering wide gain bandwidths, high brightness, and high power in a compact package.1 While many of the earliest comments about QCLs recognized not just the scientific but also the commercial importance of this technology for a wide range of applications, significant progress had to occur to transition the first cryogenically cooled QCLs into usable industrial tools.
The early adoption by the research community drove expansion in wavelength availability and tuning performance while the needs of the defense community propelled improvements in power and reliability. Today we are seeing these improvements combine to turn commercial possibilities into a reality.
Quantum cascade lasers combine many of the features of near-ubiquitous visible and near-IR diode lasers with some unique benefits. Like their visible and near-IR counterparts, QCLs are compact, solid-state devices and can be grown using conventional indium phosphide (InP) and gallium arsenide (GaAs) based epitaxial growth platforms. However, the waveguide thicknesses—not the material bandgap—determine the laser emission wavelength of the QCL, so a wide range of laser wavelengths across the mid-IR are available.
Another difference is the unipolar nature of the device where a single electron can emit multiple photons by “cascading” down the multiple quantum wells within the conduction band of the structure. This leads to high-power and high-efficiency devices, and several-watt operation has been demonstrated.
The gain bandwidth of the QCL is very broad and can cover 20% or more of the center wavelength. However, for molecular identification, imaging, and other spectroscopic applications, the output from the QCL must be modified to offer narrow bandwidths with broad tuning ranges. This can be achieved by placing the QCL in an external cavity, as Daylight Solutions has done with its ECqcL.2 The feedback from the grating selects the output wavelength from within the QC gain bandwidth, and rotation of the grating allows this output wavelength to be tuned. This design allows coverage of multiple spectroscopic features, permitting identification and characterization of molecular species.
As with much new technology, the earliest adopters of broadly tunable QCLs were in the research community. Pulsed and continuous-wave performance are both available and can be configured with a number of tuning range options including fixed, narrowly tunable (~30 cm-1), tunable (~100 cm-1), or broadly tunable (>350 cm-1) to address a variety of needs at different price points. It is also possible to tune mode-hop-free over a wide range (up to 120 cm-1). Wavelength choices have expanded from their original introduction and wavelengths as low as 3.2 µm (important for the C-H stretch region) to greater than 12 µm have been demonstrated. Finally, output powers in excess of 400 mW are available.
Another early adopter of the technology was the military. Defense applications of QCLs have been covered recently and include IR countermeasures, target illumination, and standoff detection of explosives.3 These applications have driven the development of ruggedized laser systems able to operate in severe temperature and vibration environments.
These requirements include operating over a temperature range from –54°C to +71°C with simultaneous vibrational accelerations of 6 Grms from 20 Hz to 2 kHz, and surviving a shock of 20 G peak amplitude. In addition, the lasers must operate after salt water immersion and even after a lighting strike to the surrounding platform. These requirements likely exceed most commercial requirements.
The precision required for spectroscopy research applications combined with the robustness demonstrated for the military applications have opened up commercial opportunities including the detection of volatile organic compounds (VOCs) and identification of hazardous materials.
Detecting anesthetic gases
Workplace exposure to VOCs is one industrial area where the value of the wide tuning range and fast scan speeds of the external-cavity QCL can be demonstrated. The system must be able to identify and quantify a wide range of molecules, and analysis should occur in real time to identify transient hazardous exposures.
As an example, there is increasing awareness of the risks posed to hospital personnel by anesthetic gases that leak from a patient’s breathing circuit during surgery, or arise from patient exhalation during recovery.4 Analysis of this risk, and monitoring for safety, is complicated by the variety of anesthetic gases used in hospitals, coupled with the presence of other VOCs. The requirements for sensitivity and specificity in this environment have required the use of IR spectroscopy for monitoring.
To address this and other needs, Daylight Solutions developed the Swept Sensor device, which integrates several building-block technologies that include broadly tunable mid‐IR laser sources with rapid tuning, optoelectronic detectors, system-control and digital-signal-processing electronics, a multipass cell, gas handling, and optomechanics. The integrated platform has been designed to minimize power consumption, enabling its operation from conventional battery sources. It also utilizes software and firmware code to obtain and analyze absorption data and provide molecule‐specific concentrations.
As a test of the device, the sensor was used for anesthetic gas monitoring during open heart surgery on a sheep in a hospital setting. Isoflurane was used as the anesthetic gas. A side‐by‐side comparison of the Swept Sensor to the MIRAN SapphIRe Portable Ambient Air Analyzer from Thermo-Environmental Systems (Minneapolis, MN) was performed. The MIRAN SapphIRe is one of the industry-leading gas sensors for workplace VOC monitoring.
The two gas sampling wands were placed in close vicinity to each other for most of the surgery. For brief periods of time, the sampling wands were handheld for sampling air in the room, for sniffing for leaks around the equipment, and for measuring anesthesia concentrations above the operating table. Periodically, the Swept Sensor measurements were interrupted to collect a baseline for analysis.
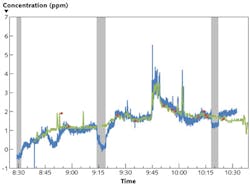
Figure 1 shows a direct comparison of the logged data of the MIRAN SapphIRe and the Swept Sensor. The Swept Sensor data have been recorded with an update rate of 1 s, and the MIRAN data set with an update rate of 5s. The two data sets correspond very well to each other; i.e., the readings of both instruments are within their respective margins of error. The data sets show larger deviations during times of active sniffing around the equipment to check for leaks (near 8:47 and 10:00), during the background measurements with the Swept Sensor, and after completion of the surgery when the instruments were wheeled out of the operating room.
Higher isoflurane concentrations were observed when sniffing close to the anesthesia delivery equipment. A spike to 5.5 ppm was observed when a surgery assistant temporarily disconnected a plastic tube from the ventilator to check something.
A second test involved the detection and monitoring of multiple anesthetic gases during a controlled release in a vacant operating room. Three common anesthetics (i.e., isoflurane, sevoflurane, and desflurane, all being highly volatile liquid anesthetics) were poured one after the other onto several tissue papers. In addition to these molecules, the Swept Sensor was also looking for halothane, which was not expected to be present in the operating room. For these tests, the sampling wand of the MIRAN SapphIRe was placed on top of the Swept Sensor while it aspired the room air through a 1 μm membrane filter.
Figure 2 shows the Swept Sensor strip charts documenting the measured concentrations of the common anesthetic gases during the release. The strip charts in each picture were simultaneously recorded at different update rates ranging from 0.1 to 90 s. The longest update rate of 90s was selected to match the update rate of the MIRAN SapphIRe.
While the actual concentrations are of less importance here, the strip charts show two important things: the need for true real-time data acquisition, and how the update rate affects the measured concentrations if the concentrations are not constant for the duration of the measurement. Figures 2a through 2e are all shown on the same concentration scale.At short update rates, we see two short transients (approximately 3 s long) of very high isoflurane concentrations (peak concentrations 250 and 300 ppm). As the update rate decreases, these high concentrations are increasingly diluted until at an update rate of 90 s only 25 ppm isoflurane are reported. This result demonstrates the need for a fast update rate to report accurate and timely concentrations of transient chemical exposures.
HazMat detection
Another industrial application highlighting the capabilities of an external-cavity QCL system is to measure and identify contents after a chemical spill or confirm contents in chemical waste containers. To demonstrate this ability, a test was performed at an industrial site where there are ongoing issues with unlabeled plastic spray bottles.
As a case in point, a chemical spill was simulated by pouring some of the content of an unlabeled spray bottle believed to contain isopropanol onto the lab counter and measuring the fumes coming off the spill with the Swept Sensor. The sensor analyzed the fumes to look for five possible vapors: n-butanol, isopropanol, methanol, acetone, and ethyl acetate. Analysis revealed that the spray bottle contained apart from isopropanol substantial amounts of methanol. We speculate that the unlabeled bottle had accidentally been refilled with the wrong solvent. These results demonstrate that the sensor can be used for quickly identifying the chemical composition of a chemical spill in a hazardous materials (HazMat) cleanup situation.
As a further example, the headspace of an empty formalin container believed to contain remainders of formaldehyde was measured with the Swept Sensor. Unexpectedly, total xylene concentrations up to 1500 ppm were found in addition to water absorptions. It appears that the empty container had been rinsed with xylene to prepare it for disposal.
A closer inspection of a spectrum recorded during the headspace measurement does show that formaldehyde signatures are clearly present. Based on the observed peak intensity at 1121 cm‐1, the formaldehyde concentration was estimated to be approximately 100 ppm. The sensor has thus successfully identified the true chemical composition of the contents in a chemical waste container.
Since the first QCL was demonstrated in 1994, much progress has been made to bring these devices into industrial applications. The research community drove improvements in wavelength options and tuning performance while the defense community drove greater power and reliability. These needs are combined in commercial applications, and as shown by the examples here, quantum cascade lasers have made the transition from laboratory demonstrations to commercially useful products.
REFERENCES
1. J. Faist et al., “Quantum Cascade Laser,” Science, 264, 553 (1994).
2. M.J. Weida, P. Buerki, E. Takeuchi, and T. Day, “External-cavity QCLs broaden capabilities for molecular detection,” Laser Focus World, 46, 4, 58-62 (April 2010).
3. G. Overton, “IR countermeasures aim for safer flights,” Laser Focus World, 47, 8, 35–43 (August 2011).
4. “Waste Anesthetic Gases – Occupational Hazards in Hospitals,” DHHS (NIOSH) Publication No. 2007–151, September 2007; http://www.cdc.gov/niosh/docs/2007-151/pdfs/2007-151.pdf.
Bob Shine is senior director of commercial business development, Peter R. Buerki is applications scientist, and Tim Day is CEO and CTO of Daylight Solutions, San Diego, CA rshine@daylightsolutions; www.daylightsolutions.com.