ByHusain Imam
Now widely embraced in many fields, supercontinuum sources offer life scientists unprecedented flexibility. The technology is enabling both research and clinical applications, including the industry's first commercial optical coherence tomography (OCT) system capable of cellular-level resolution.
From the advent of the supercontinuum laser, makers and users of systems for life sciences have been drawn to the powers of this light source that is "broad as a lamp and bright as a laser." Supercontinuum light provides the advantages of a laser, with its high power, diffraction-limited output, and as a lamp, with its extreme broadband spectrum covering more than two octaves of optical frequency. Today, a number of vendors are providing supercontinuum sources that span from the UV to the infrared (IR)—sources that give researchers and clinicians flexibility in illuminating, interrogating, and exciting biological materials and chemicals to further understand their complex functionality.
Each of the different optical properties that can be used to examine a specimen requires a different region of the wavelength spectrum (see Fig. 1). In fluorescence, visible wavelengths are typically required to excite fluorescent dyes and labels, whereas in optical coherence tomography (OCT), near-infrared (NIR) wavelengths are preferred due to their deeper penetration into tissue. The availability of a large spectral range gives scientists great flexibility in choosing the most appropriate mode or multiple modes of interrogating the sample with light. Importantly, supercontinuum light is diffraction-limited, allowing high-resolution analysis at the cellular level. Furthermore, because supercontinuum sources are pulsed, they add time as a discriminator, which is important in lifetime studies.
Ex vivo applications: microscopy
A good example of exploiting supercontinuum light for life sciences is confocal microscopy. Leica Microsystems' (Wetzlar, Germany) SP8x confocal microscope system employs the SuperK supercontinuum source (NKT Photonics; Birkerød, Denmark) to drive fluorescence lifetime imaging microscopy (FLIM), Förster resonance energy transfer (FRET), and gated stimulated emission depletion (g-STED). Here, the availability of any wavelength in the visible spectrum allows optimization of the fluorescence signal by tuning the wavelength to its maximum absorption. If multiple dyes are used, wavelengths can be optimally tuned to ensure maximum contrast and minimal cross-excitation. The pulsed nature of the source, together with the broad choice of wavelength, adds another dimension for FLIM, FRET, and g-STED. For lifetime applications such as time-correlated single photon counting (TCSPC), the pulse width of the excitation wavelength is much shorter than the lifetime of the sample, and there is enough pulse energy to provide the excitation.
The pulse widths of the SuperK are narrower than commonly used pulsed diodes, and the powers per wavelength are much larger (see Fig. 2). Confocal fluorescence images of triple-stained cells show the power of multiple-wavelength tuning, especially in the orange, where laser availability is a challenge. For each of these dyes, pulsing enables measurement of lifetime decay.
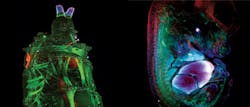
A relatively newer technique, light sheet microscopy, is also beginning to benefit from supercontinuum technology. Recently, LaVision BioTec (Bielefed, Germany) installed in the field a number of Ultramicroscopes (see "The planar truth about light-sheet microscopy," p. 40) driven by SuperK supercontinuum sources. The principle of the technique is to illuminate the sample from the side with a very thin sheet of light (5–40 μm × 0.1–10 mm) and image the excited plane using a microscope perpendicular to the light sheet. By moving the sample through the light sheet, one can build up a stack of images to create a three-dimensional (3D) rendering of the sample (see frontis). The technique is ideal for viewing tissue structures and morphology and allows the imaging of whole mouse brains, lymph nodes, and tumors in a short space of time, which is important in pharmaceutical research. Again, the broad visible spectrum for fluorescence excitation, high power available in each wavelength, and diffraction-limited output allow the generation of light sheets at any wavelength in the visible and NIR spectrum.
In vivo: retinal imaging and psychophysics
In addition to ex vivo applications, supercontinuum sources benefit a growing range of uses in vivo. For instance, retinal imaging yields information that can help in the diagnosis of glaucoma, macular degeneration, and other retinal disorders. Also, it allows the investigation of psychophysical behavior involving the eye; in other words, how the eye and brain interact (visual neuroscience). Because the retina and optic nerve comprise an extension of the neural or brain tissue, interrogation of the eye can yield information about early neurological disorders or early signs of disease.
Retinal images with high spatial resolution are necessary for viewing structures such as blood vessels and individual cones and receptors located on the retina. An adaptive optics scanning laser ophthalmascope (AOSLO)—basically a confocal microscope for imaging eyes—uses laser light of differing wavelengths to illuminate the retina and adaptive optics alters the wavefront to compensate for any aberrations due to the cornea and lens (see Fig. 4).
As in conventional confocal microscopy, images can be obtained at different focal planes to examine the layers of the retina. Using multiple wavelengths from a supercontinuum source enables stimulation of different parts of the retina when employing fluorescence (for retinal angiography) and retinal spectroscopy (to measure oxygen content in blood flow within the eye)—which is being researched now for eventual clinical application in diagnosis of diseases such as glaucoma.
The high coherence length of traditional lasers creates speckles that severely limit the ability to resolve microscopic retinal features (see Fig. 3b). Being able to select any wavelength within the visible spectrum allows testing retinal circuitry and health at the cellular scale. Moreover, the ability to change coherence length by simply selecting output bandwidth will enable testing some of the more recent hypotheses about how the vision process starts in the outer segments of a photoreceptor. The current thinking is that the outer segments behave like interferometers and that the signal reflected back to the pupil changes over time according to the length of the segments. Using multiple wavelengths simultaneously might allow measurement of the absolute length of these cells in one snapshot (see Fig. 3a), potentially leading to a powerful diagnostic tool.
An example is in visual neuroscience. Specific wavelengths (545 and 710 nm), difficult to obtain using conventional lasers, maximize a differential response from stimulating "red" and "green" cone photoreceptors in the eye. Only at 545 nm are red and green cones equally sensitive; at 710 nm, their sensitivities are maximally different. Supercontinuum technology can facilitate psychophysical measurements—for example, mapping the cone types in humans—by flashing these different wavelengths onto single cones of a patient and asking him/her what color he/she saw. Furthermore, broader bands—say, 10–20 nm—reduce interference problems and produce much cleaner images, which would not be possible with narrow-band lasers. This capability allows the identification of cone types with simultaneous physiological recordings.
And cellular-level OCT
Optical coherence tomography (OCT) is rapidly expanding as a technique for noninvasive in vivo analysis of cross-sections of tissue or organs. It can be described as the optical analog of ultrasound, using optical waves instead of sound waves. As with ultrasound, it is the time delay between optical waves reflected from different depths in the tissue that enables 3D imaging of the sample. Consequently, the change of wavelengths of a few millimeters (sound) to now about 1 μm (light) leads to an increase in resolution; 1–10 μm for OCT compared to greater than 150 μm for ultrasound.
Typically, for spectral-domain OCT (SD-OCT),2 a broadband, incoherent source is required. In SD-OCT, the axial resolution of the imaging will be dictated by the coherence length of the source. The lower the coherence, the finer images can be obtained over thinner sections of sample, as the short coherence acts a gate. Light sources with large bandwidth have low coherence length (see Fig. 5).
The wavelengths required for OCT are important because for in vivo penetration of tissue, optical scattering and autofluorescence are detrimental. The diagnostic or therapeutic window lies at 700–1300 nm. Mainly two wavelengths in this range are used in OCT: For ophthalmology, 800 nm is used primarily because light absorption of blood in vessels enhances the images. For deeper tissue imaging in skin or for endoscopy, 1300 nm is preferred due to its greater penetration depth.
The most widespread clinical use of OCT is in ophthalmology, where the internal imaging of the retinal layers is carried out. Here, increased resolution enables early diagnosis—and more effective treatment—of age-related macular degeneration (AMD) and diabetic retinopathy.
Superluminescent diodes (SLEDs) have been the traditional source of choice for SD-OCT. However, for high resolution, SLEDs can deliver large bandwidth only when multiple narrower-band SLEDs are combined, resulting in very uneven spectral shape and low power. High-resolution OCT with supercontinuum has been demonstrated,3 but there has always been a question of the noise performance of supercontinuum sources limiting the imaging quality. Bioptigen (Research Triangle Park, NC), a pioneer of OCT for ophthalmology, experimented with supercontinuum technology in an effort to obtain higher-resolution images of the macula (see Fig. 6).
Based on these results, Bioptigen has introduced the new Envisu XHR OCT system, using the company's commercially available SD800 XHR 300 nm spectrometer to allow full utilization of the wide bandwidth of the SuperK system. The result is the first commercial system to reach 1 μm axial resolution, offering researchers true cellular-level in vivo optical histology (see Fig. 7).
For some applications, deploying different optical techniques or modes simultaneously provides a great advantage. An example is ophthalmology—as described above, one supercontinuum source could enable both retinal imaging and OCT, allowing the use of both modalities to obtain both structural and functional information. For instance, the work of Zhang et al. used supercontinuum light not only for ophthalmic OCT, but also for oximetry to provide more information of the pathophysiology of early disorders.4
Biomedical application of supercontinuum light is in its infancy, but in many cases the technology enables greater flexibility, increased resolution, and cost-effective multimodal implementation. High-power supercontinuum sources are finding new applications, which will be exciting to observe as they mature into preclinical and clinical applications.
References
1. L.C. Sincich et al., "An adaptive optics retinal microstimulator for color vision studies," Society for Neuroscience Abstracts (2011).
2. D.D. Sampson and T. R. Hillman, "Optical coherence tomography, lasers and current optical techniques in biology," ESP Comprehensive Series in Photosciences, 481–571, Cambridge, England (2004).
3. L. Liu et al., Nat. Med., 17, 1010–1014 (2011).
4. J. Yi, Q. Wei, W. Liu, V. Backman, and H. F. Zhang, Opt. Lett., 38, 11, 1796–1798 (2013).
HUSAIN IMAM, Ph.D., is regional manager for NKT Photonics, Morganville, NJ; e-mail: [email protected]; www.nktphotonics.com.