Mid-infrared Spectroscopy/Bioimaging: Moving toward MIR optical biopsy
ANGELA B. SEDDON, BRUCE NAPIER, IAN LINDSAY, SAMIR LAMRINI, PETER M. MOSELUND, NICK STONE, and OLE BANG
Limited availability of tests to diagnose cancer in its early stages has contributed to an unfortunate prevalence of late-stage diagnoses and metastatic spread. For this reason, emerging technologies that promise early diagnosis constitute a key focus of research. Mid-infrared imaging (MIR), with its ability to enable in vivo medical diagnosis, is particularly interesting. In fact, the European Commission provides support for a major effort to develop the technology through its Framework Seven (FP7) project called MINERVA (MId- to-NEaR- infrared spectroscopy for improVed medical diAgnostics).
Why MIR?
In 1800, Sir William Herschel used a thermometer to analyze sunlight passing through a prism, and detected "heat rays" beyond the red of the visible rainbow.1 Discovering a "rainbow of colors" in the sunlight at shorter frequencies than those visible to the naked eye, he named the infrared (IR; Latin for "beneath red") region with far-reaching implications. Since then, we have come to divide the invisible IR rainbow into three light frequency regions: near-infrared (NIR), mid-infrared (MIR), and far-infrared (FIR) (see Fig. 1).
An interesting characteristic of the MIR is that oscillation frequencies of lightwaves in this region match the frequencies of characteristic vibrations of molecular bonds. Thus, bond vibration increases in amplitude when (resonantly) absorbing MIR light of the same frequency. Shining a bright MIR rainbow of light at a molecular sample and collecting the light after its interaction with a sample reveals that some MIR frequencies are diminished in brightness. That is, those frequencies have gone to stimulate particular molecular vibrations in the sample. This technique is called MIR spectroscopy and for any given sample, the complex pattern of diminished and undiminished light frequencies is called the characteristic MIR spectrum of the material (see Fig. 2).MIR molecular spectroscopy of cells and tissue
The MIR spectrum of a sample enables us to detect, identify, and even quantify its molecular makeup. Thus, we can sense numerous molecular gases, liquids, and solids, including biological tissues such as human cells, though the technique is also applicable to greenhouse gases; ground, water, and air pollutants; pharmaceuticals; toxic agents; narcotics and explosives; food and beverages; oil, oil products, and plastics; and more.
All living tissue is composed of cells, which comprise biomolecules2—DNA is one example. Biomedical MIR absorption spectroscopy on cells and tissue may be conducted either in vitro (in the laboratory) or ex vivo (in the laboratory, but with minimal alteration of natural conditions), as opposed to in vivo (in a live body). To understand the MIR spectroscopy of a collection of biomolecules as a living entity is a compelling notion!
Biomedical spectroscopist Max Diem, a professor at Northeastern University (Boston, MA), has been in the vanguard of in vitro and ex vivo MIR spectroscopy of human cells and tissue. His seminal paper3 was critical of some prior work being too simplistic in using MIR absorption spectroscopy to detect malignant, as opposed to normal, cells and tissue. MIR spectral changes between diseased and normal tissue are subtle, and statistical analysis (principal component analysis or clustering techniques) of multiple MIR spectra are in fact required to confirm these differences.
Diem has highlighted that MIR spectroscopy of cells and tissue can potentially provide an immense amount of information on cellular composition, packing of cellular components, organ and cell architecture, metabolic processes, and the absence/presence of disease. Most importantly, he was the first to recognize that the spectroscopic information revealed by cells and tissue may revolutionize the way that pathology is performed in the 21st century. Pathology is the study and diagnosis of disease through examination of organs, tissue, cells, and bodily fluids.
Currently, ex vivo biomedical MIR molecular vibrational absorption spectroscopy is accruing evidence of the ability to distinguish excised diseased tissue from normal tissue.4,5 MIR spectra are collected using a microspectrometer, a desktop instrument combining MIR microscopy with Fourier transform (FT) MIR spectroscopy (see Fig. 2). The MIR rainbow light source is usually either a synchrotron MIR beam or conventional blackbody-type source (e.g., GloBar). MIR spectral collection across the lateral dimension of the tissue sample in imaging mode uses a focal-plane array (FPA)-type MIR mercury cadmium telluride (MCT) detector to collect directly a pixelated image based on the averaged MIR spectral absorption of tissue captured per pixel to obtain a visual representation of the molecular makeup of a tissue sample in terms of false color maps. MIR micro-spectroscopic imaging is widely recognized as nondestructive, label-free, and highly sensitive, and is applied particularly ex vivo in cancer research and diagnosis on excised tissue. State-of-the-art MIR MCT FPA imaging of excised tissue from the MINERVA Project is shown in Fig. 3.5Today's gold standard for cancer diagnosis is histopathology, which involves microscopic study (using visible light) dye-stained cells and tissue. Most major hospitals are equipped with a "path lab," where excised tissue, taken from the patient, is frozen, microtomed into slices <10 μm thick, and then individually mounted in sequential order on microscope slides and stained with hematoxylin and eosin (H&E) dyes to reveal cell and tissue morphology (shapes). A pathologist's examination of the processed tissue informs diagnosis and treatment planning. Histopathology is time- and labor-intensive, highly dependent on the judgment of the pathologist, and involves patient discomfort and stress while awaiting the biopsy results.
In vivo imaging with MIR
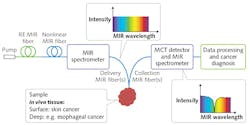
The goal of the MINERVA project is, instead, to achieve in vivo MIR molecular vibrational spectroscopy. MIR optical biopsy in situ in the body aims to provide fast, real-time cancer diagnosis to reduce patient discomfort. For example, MIR endoscopic monitoring of cancer margins during surgery (including during MIR fiber laser surgery) would involve portable, inexpensive MIR photonic systems capable of being used in either a hospital/clinic setting or a primary-care practice. This new paradigm will be enabled through focused development of MIR photonic devices and systems that are robust, functionally designed, safe, compact, cost-effective, and based on active and passive MIR optical fiber.7-9
MIR cell and tissue studies have typically involved a benchtop FT MIR spectrometer designed to keep the weak blackbody-type MIR source, sample, and MIR detector in close enough proximity that MIR photons can complete the optical circuit. Alternatively, excised cell and tissue samples are taken to a non-portable, bright synchrotron MIR source. Key to development of in vivo systems are MIR light sources that are broadband, very bright, and portable.
Within the MINERVA project, the MIR Photonics Group at the University of Nottingham (England), in conjunction with researchers at Technical University of Denmark (DTU; Copenhagen, Denmark), has demonstrated extreme broadband supercontinuum (SC)-generated light 1.4–13.3 μm in a specially engineered, high-numerical-aperture MIR optical fiber (see Fig. 4).9,10 These active MIR fibers, exhibiting broadband supercontinuum lasing, have the potential to rival the brightness of even synchrotron-produced MIR light—thus offering, for the first time, the possibility of a bright MIR wideband source in a portable package. The work has been corroborated by others.11Availability of low optical-loss silica glass fiber has enabled the development of Raman scattering for in vivo MIR optical imaging, another emerging clinical technique. Raman and MIR absorption are both vibrational spectroscopies, governed by different quantum mechanical selection rules. Because the MIR approach is based on direct fundamental vibrational molecular absorption, it is potentially more sensitive, with excellent contrast for clinical application. While the Raman effect is intrinsically weak, its spectral output can complement that of MIR absorption, so together the approaches can work synergistically.
REFERENCES
1. W. Herschel, Philos. Trans. R. Soc. Lond., 90, 284–292 (1800).
2. H. H. Mantsch, foreword to "Biomedical applications of synchrotron infrared microspectroscopy: A practical approach," RSC Analytical Spectroscopy Monographs, 11 (2011).
3. M. Diem, S. Boydston-White, and L. Chiriboga, Appl. Spectrosc., 53, 4, 148A–161A, 1999.
4. H. Sreedhar et al., J. Vis. Exp., 95, 52332 (2015); doi:10.3791/52332.
5. N. Jayakrupakar, G. R. Lloyd, N. Shepherd, and N. Stone, "High-resolution FTIR imaging of colon tissues for elucidation of individual cellular and histopathological features," Analyst (accepted for publication in 2015).
6. A. B. Seddon, "Mid-infrared photonics for early cancer diagnosis," Proc. ICTON, doi:10.1109/icton.2014.6876433 (2014).
7. A. B. Seddon, Int. J. Appl. Glass Sci., 2, 3, 177–191 (2011).
8. A. B. Seddon, Phys. Status Solidi B, 250, 5, 1020–1027 (2013).
9. R. Petersen et al., Nature Photon., 8, 830–834 (2014).
10. G. R. Steinmeyer and J. S. Skibina, Nature Photon. News and Views, 8 (2014).
11. Y. Yu et al., Opt. Lett., 40, 6, 1081–1084 (2015).
12. A. B. Seddon, Z. Tang, D. Furniss, S. Sujecki, and T. M. Benson, Opt. Express, 18, 25, 26704–26719 (2010).
13. Z. Tang et al., Opt. Mater. Express, 5, 4, 870–886 (2015).
14. Z. Tang et al., Opt. Mater. Express, 5, 8, 1722–1737 (2015).
Angela B. Seddon is professor with the Mid-Infrared Photonics Group at the University of Nottingham (England); Bruce Napier is researcher with Vivid Components Ltd. (Paderborn, Germany); Ian Lindsay is with Gooch & Housego Ltd. (Somerset, England); Samir Lamrini is a laser researcher with LISA laser products OHG (Katlenburg-Lindau, Germany); Peter M. Moselund is a research engineer with NKT Photonics A/S (Birkerød, Denmark); Nick Stone leads the biomedical spectroscopy team at the University of Exeter (England); and Ole Bang is professor and group leader at DTU (Technical University of Denmark).



![FIGURE 4. Achieving record extreme broadband supercontinuum-generated light in a specially engineered MIR optical fiber involves input pump spectrum (dashed line) and spectral profile at maximum pump power (solid line), showing broad, flat supercontinuum (1.64–11.38 μm at -20 dB) followed by a strong spectral peak extending the spectrum all the way to 13.3 μm (a).9 Spectral evolution with increasing pump peak power shows a gradual redshift of a distinct spectral peak at the long-wavelength edge, plus corresponding formation and blueshift of dispersive waves (b). Fiber output near-field beam profiles—for all wavelengths of input pump spectrum (c) and beam profile for wavelengths >7.3 μm only (d)—show that long wavelengths are still confined to the core. This exceeds previous record values by factor of two [10]. FIGURE 4. Achieving record extreme broadband supercontinuum-generated light in a specially engineered MIR optical fiber involves input pump spectrum (dashed line) and spectral profile at maximum pump power (solid line), showing broad, flat supercontinuum (1.64–11.38 μm at -20 dB) followed by a strong spectral peak extending the spectrum all the way to 13.3 μm (a).9 Spectral evolution with increasing pump peak power shows a gradual redshift of a distinct spectral peak at the long-wavelength edge, plus corresponding formation and blueshift of dispersive waves (b). Fiber output near-field beam profiles—for all wavelengths of input pump spectrum (c) and beam profile for wavelengths >7.3 μm only (d)—show that long wavelengths are still confined to the core. This exceeds previous record values by factor of two [10].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/02/content_dam_lfw_print_articles_2016_02_1602lfw_bow_sed_f4.png?auto=format,compress&fit=max&q=45?w=250&width=250)