Photoacoustics/Biomedical Imaging: Photoacoustic imaging progresses toward medical diagnostics
CHRIS JUN HUI HO and MALINI OLIVO
Optoacoustic imaging has seen tremendous progress over the past decade. Advances in image reconstruction algorithms, laser and transducer hardware, and contrast agents have helped drive this progress, as have demand in such applications as oncology, dermatology, and therapy monitoring. As evidence of growth in this field, a new open-access journal, Photoacoustics,1 was launched in 2013. In this article, we attempt to provide a snapshot of recent advances, especially in the context of clinical translation.
Contrast agents
Among the varieties of optoacoustic contrast agents available are fluorophores, gold and silver nanoparticles, carbon-based compounds, gene reporters, and "smart" activatable probes. Of these, certain photosensitizers, and the FDA-approved dye indocyanine green (ICG), have already been used in clinical trials—and thus may lead the march toward clinical translation.Photosensitizers offer such advantages as theranostic capabilities, preferential tumor uptake, and clinical relevance. In addition, the limitation of low fluorescence quantum yield in some photosensitizers becomes an advantage for optoacoustic imaging since such fluorophores can provide strong optoacoustic signals because of competition between fluorescence and heat generation in energy relaxation pathways. Thus, scientists have been exploring the feasibility of various types of photosensitizers as potential optoacoustic contrast agents, which include methylene blue, porphyrins, squaraines, BODIPYs, phthalocyanines, and napthalocyanines.
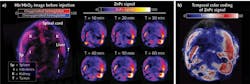
For instance, Olivo and team2 evaluated and compared the optoacoustic activity of five representative photosensitizers-zinc phthalocyanine (ZnPc), protoporphyrin IX, 2,4-bis [4-(N, N-dibenzylamino)-2,6-dihydroxyphenyl] squaraine, chlorin e6, and methylene blue in phantoms using a multispectral optoacoustic tomography (MSOT) system. Of these, ZnPc exhibited the strongest optoacoustic signal. Then, they moved on to in vivo imaging, administering this compound into a mouse xenograft model and monitoring its biodistribution and tumor uptake via optoacoustic imaging. As shown in Fig. 1, upon intravenous injection, this compound started accumulating at the tumor and reached a peak tumor uptake at 1 h, thus making this timepoint ideal for localized photodynamic therapeutic treatment. A follow-up study3 compared the optoacoustic activity of three phthalocyanine photosensitizers—phthalocyanine tetrasulfonic acid (PcS4), Zn(II) phthalocyanine tetrasulfonic acid, and Al(III) phthalocyanine chloride tetrasulfonic acid—and found that PcS4 exhibited the strongest optoacoustic signal both in phantoms and mice.
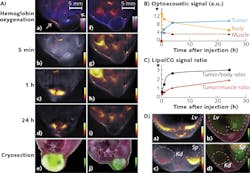
Ntziachristos and team have used ICG to improve dynamic contrast, for imaging its passive uptake in tumors and various organs such as the liver and kidneys. Recently, for instance, they incorporated ICG in PEGylated liposomes (LipoICG)4 and imaged the LipoICG distribution within the tumor with high sensitivity (see Fig. 2). Other ICG conjugates, including ICG-embedded nanoparticles and ICG-conjugated single-wall carbon nanotubes, have also been developed for optoacoustic imaging.
Light sources and detectors
In photoacoustic imaging, a light source generates nanosecond pulses with energy that is absorbed by the tissue. Such pulsed laser sources should fulfill the American National Standards Institute (ANSI) maximum permissible exposure (MPE) limit of 20 mJ/cm2 for use in patients, while simultaneously delivering homogeneous surface illumination on target tissue. High pulse repetition rates can minimize motion artifacts especially during handheld imaging, but they limit the energy per pulse, as well as the MPE.
Piezoelectric detectors are typically used to measure the output because of their superior sensitivity and robustness, although more complex detectors such as broadband10 and capacitive micromachined-based11 ones are also being developed.
Recent advances in laser, transducer technology, and computing power enable the reduction in size and cost of conventional photoacoustic (PA) systems into an integrated handheld probe-based system about the same size as those used in portable ultrasound systems. For instance, scientists12, 13 have developed video-rate, handheld-based MSOT imaging systems, which can be used to resolve tissue morphology and vasculature in human volunteers. Others have designed handheld probes that can be connected to existing ultrasound systems for real-time, dual-modality ultrasound/optoacoustic imaging.14 Such real-time imaging offers great diagnostic potential in applications such as cardiovascular diseases, lymphatic disorders, inflammation, and oncology.
Applications
Within the near-infrared (NIR) region where light absorption in tissues is relatively weak, endogenous chromophores with strong light absorption, such as hemoglobin (Hgb), melanin, and lipids, can be exploited to provide intrinsic contrast. For instance, the relative concentrations of oxy- and deoxyhemoglobin can be used to estimate oxygen saturation in blood vessels and tissues. Melanin distribution can be used for imaging melanoma and hair follicles, whereas lipid content can be used for imaging atherosclerotic plaques and sebaceous glands. In addition, exogenous contrast agents can be administered to improve contrast or offer target specificity with ligand bioconjugation. However, as mentioned earlier, ICG is the only NIR dye, which is FDA-approved for clinical use currently.
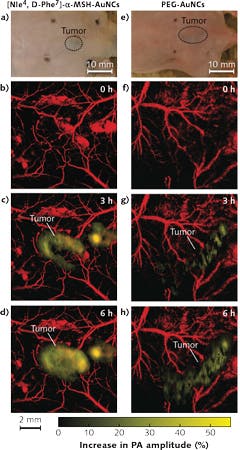
Optoacoustic imaging has been used in oncology applications to image the blood vasculature and oxygenation, and to monitor angiogenesis in tumors. Thanks to the presence of dense vasculature at the tumor site compared to that of normal tissues, optoacoustics can be used for tumor detection based on Hgb contrast. Exogenous contrast agents such as fluorescent dyes and gold nanoparticles can also be used to improve imaging contrast. For instance, Wang and team5 developed gold nanocages (AuNCs) bioconjugated with α-melanocyte-stimulating hormone, for active-targeted imaging of melanomas (see Fig. 3). At the same time, they were able to monitor the physiological state of the melanoma longitudinally by measuring oxygen saturation in the surrounding blood vessels.
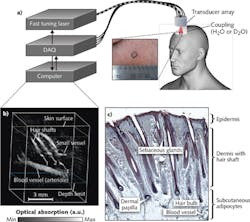
Pilot studies are underway to assess the efficacy of optoacoustic imaging for melanoma characterization in patients.6,7 Melanoma staging is based on tumor depth, which is typically examined by an experienced pathologist via biopsy and histology. Optoacoustic imaging can provide an alternative noninvasive technique for rapid screening of melanomas in patients, with real-time feedback to clinicians during an in-patient procedure.Optoacoustic imaging can also be applied to skin studies such as transdermal water absorption and drug delivery, skin disorders, and wound and burn healing. It can be used to image the hair papilla, which is useful for the diagnosis of hair and scalp diseases such as alopecia areata, female androgenic alopecia, monilethrix, and Netherton syndrome. Currently, the gold standard of assessment typically involves dermatoscopy and analysis of hair biopsy samples—an invasive procedure. MSOT is capable of noninvasive diagnostics: Imaging the hair follicle structure, the oxygenation status of the capillary bed surrounding the hair papilla, as well as the lipid composition of the pilosebaceous unit (see Fig. 4).8 Optoacoustic imaging has also proven useful for imaging skin morphology and blood oxygenation in chronic wounds, ulcers, and burns, which is critical in monitoring the healing process. For instance, raster-scanning optoacoustic microscopy (RSOM)9 can be used to perform high-resolution imaging of vasculature in the dermis to an axial resolution of 4 μm.
Future directions
Heralding the future, Seno Medical Instruments recently received the first CE-mark approval for an optoacoustic system targeting breast cancer imaging. The system represents a significant milestone in the move towards clinical translation of photoacoustics. It also represents a milestone in clinical care, because unlike most modalities, it is based on functional—not anatomical—imaging.
The system illuminates large regions of tissue with short laser pulses, operating on the principle that tumors absorb light preferentially compared to normal tissue. The tumors become slightly heated, while transient, thermoelastic expansion causes them to emit pressure (acoustic) waves detectable by an array of wideband ultrasonic sensors positioned around the periphery of the breast held within a probe. And because laser optoacoustic imaging can identify tumors as small as 2 mm and visualize submillimeter structures, it is promising for early detection—before biological advancement is able to result in metastasis.
The technique and underlying physics enable NIR illumination to produce image contrast that reveals Hgb concentration and oxygen saturation, both of which have direct relevance to tumor pathophysiology. While angiogenesis itself is insufficient to differentiate malignant from benign lesions, the combination of Hgb concentration and relative oxygenation has good diagnostic accuracy.
Algorithms analyze signals from the sensors and assemble them into high-contrast, high-resolution images that depict lesions in striking color. Because malignant tumors possess increased microvasculature but deplete oxygen at a higher rate than benign growths, the difference is apparent: Deoxygenated blood results in brighter images in the presence of shorter light wavelengths. The reconstruction algorithms can determine spatial location of optical absorbers from the time-domain data.
The future of optoacoustic imaging is promising, as advances in contrast agents, light sources, sensors, systems, novel image reconstruction, and highly accurate spectral unmixing algorithms are being implemented.15, 16 Overall, further developments will bridge the gap between scientific potential and clinical relevance, and determine whether the technique can offer novel insights in disease diagnosis, progression, and therapeutic responses to treatment.
REFERENCES
1. See http://www.journals.elsevier.com/photoacoustics.
2. C. J. H. Ho et al., Sci. Rep., 4, 5342–5347 (2014).
3. A. B. E. Attia, G. Balasundaram, W. Driessen, V. Ntziachristos, and M. Olivo, Biomed. Opt. Express, 6, 591–598 (2015).
4. N. Bézière et al., Biomaterials, 37, 415–424 (2015).
5. C. Kim et al., ACS Nano, 4, 8, 4559–4564 (2010).
6. I. Stoffels et al., J. Nucl. Med., 56, 40 (2015).
7. A. Breathnach et al., Proc. SPIE, 9303, Photonic Therapeutics and Diagnostics XI, 930303 (Mar. 9, 2015); doi:10.1117/12.2078309.
8. S. J. Ford et al., J. Invest. Dermatol. (accepted 2015).
9. M. Omar, M. Schwarz, D. Soliman, P. Symvoulidis, and V. Ntziachristos, Neoplasia, 17, 2, 208–214 (2015).
10. C. Zhang, T. Ling, S.-L. Chen, and L. J. Guo, ACS Photon., 1, 11, 1093–1098 (2014).
11. S.-R. Kothapalli et al., IEEE Trans. Biomed. Eng., 59, 5, 1199–1205 (2012).
12. A. Buehler, M. Kacprowicz, A. Taruttis, and V. Ntziachristos, Opt. Lett., 38, 9, 1404–1406 (2013).
13. X. L. Deán-Ben and D. Razansky, Photoacoustics, 1, 68–73 (2013).
14. K. Daoudi et al., Opt. Express, 22, 21, 26365–26374 (2014).
15. C. Lutzweiler and D. Razansky, Sensors, 13, 7345–7384 (2013).
16. G. P. Luke, S. Y. Nam, and S. Y. Emelianov, Photoacoustics, 1, 2, 36–42 (2013).
Chris Jun Hui Ho, Ph.D., is a Research Fellow with the Singapore Bioimaging Consortium (SBIC), with an Associate Lecturer appointment at SIM University, Singapore. Prof. Malini Olivo is head of the Bio-Optical Imaging Group at Singapore Bioimaging Consortium, Agency for Science, Technology and Research, Singapore.