Point-of-care Disease Diagnosis: A breath screen for active tuberculosis at the point-of-care
DENNIS CAMILLERI
Driven by various social factors, a number of "old" diseases have recently re-emerged to significantly threaten world health. Of particular concern is the increase in tuberculosis (TB), a highly contagious disease spread through the air when those infected cough, sneeze, or spit. According to statistics published by World Health Organization (WHO), 8.8 million active cases are diagnosed each year, and almost 2 million die. Antibiotic resistance has resulted in multidrug-resistant (MDR TB) and extra-multi drug-resistant (XDR TB) forms of the disease, both of which are highly contagious and very expensive to treat. While most cases occur in the world's poorest countries, no region is free from the threat-infection levels are rising in Western Europe, and health organizations in North and South America are taking proactive control measures to minimize TB's spread.
The addressable market opportunity for rapid screening and diagnosis of infectious diseases is substantial and rising, with global spend on TB screening alone >$1.5 billion-and with approximately 193 million TB screening tests forecasted to take place annually by 2020.1
While new and better medicines are important, early diagnosis is the critical starting point. This—along with effective treatment—leads to a better prognosis for the patient and reduces potential for spread of the infection.
Control of TB—of which early diagnosis is a key part—enables significant lowering of healthcare costs. The first step in diagnosis is rapid and accurate screening that is ideally low-cost and capable of administration with minimal training. Unfortunately, today's technologies for TB detection fits none of these criteria, and so a better solution is urgently needed.
An optical breath analyzer
A noninvasive medical device incorporating a range of technologies allows for full portability and almost instantaneous TB test results. It can be assembled without the need for clean room conditions, and used by non-medical personnel. As a result, the technology is particularly suitable for use where cost is a key factor to implementing treatment.
Development of the device required a combination of extensive experience in medical diagnosis and knowledge of integrated photonics, comprising optical design, biophotonics, and robust industrial design capable of production scalable to high volume.
The TB Breathalyzer, patented by Rapid Biosensor Systems, consists of a plastic cough sample collection tube (10 cm in length and 3.5 cm in width) containing a bio-optical sensor covered in a patented bio-chemical coating formulated to react with the TB bacilli. The coating is dry and no mixing of additional fluids is required.
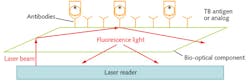
After a patient coughs into the tube, the technician closes the plunger and rotates the upper half of the tube, thereby engaging the tube's dynamics to create a disc of cough sample and position it over the biosensor. Closing the tube effectively seals the Breathalyzer and protects the technician from contact with the cough sample. The tube is then inserted into the optical reader and twisted to switch on the unit. The reader takes two minutes to measure the fluorescent light signal change (see figure).
The reader analyzes a patient's sample by performing a displacement assay based on evanescent wave technology, an established biochemical process that is proven and fast (see sidebar, "Bio-optical processing"). The reader unit incorporates a diode laser that interrogates the bio-chemical coating carrying the sample. The coating contains analogs labeled with a fluorescent material. In the displacement assay process, the TB antigen displaces fluorescence-coated analogs and bonds more strongly to antibodies, causing a reduction in the fluorescence signal after excitation by the laser. The laser detects this signal change as a decrease in fluorescent light near the surface, as the labeled analogs move away from it and the unit returns a positive result.
Reading and analysis of the sample takes approximately two minutes and, from start to finish, the entire screening process takes about four minutes. After use, the sample tube is destroyed and the reader is set to analyze the next sample. There is no mutual interface that might contaminate any future sample.
Product design
Rapid Biosensor Systems implemented a phased approach to product design starting with proof of principle and followed by design iteration, prototyping, and field testing.3 There was little point in following a "blue sky" technology idea that could not be transferred into a robust medical device able to be easily manufactured at low cost and in high volume. The idea was to develop a product able to exceed both technical performance expectations and commercial targets advised by the WHO for TB screening—and ultimately enable a screening test for active TB in the millions of units.
Biological sample processing can involve a complex set of materials and interactions that need to be harnessed and implemented in a practical hardware solution. For example, any device that includes a biochemical coating and optical components will involve optical scatter as light interacts at several interfaces. The TB Breathalyzer and reader were modeled as an integrated optical system, and its components were chosen to optimize and balance high performance with low manufacturing cost. The tube design takes care of the fluid mechanics associated with collection of cough samples, and the reader is designed to quickly detect low levels of fluorescent light generated by the assay. Selection of photonics components, including laser, optics, and filters, was undertaken after we optimized the design using optical design software. We did not use any custom-designed photonics components and instead were able to keep the bill of materials low by selecting standard components. But in no way did this fact impact the ability to deliver excellent performance.
An important aspect to producing new innovative solutions to medical testing is that the tests have to be easily tolerated by the patient. The Breathalyzer test is well tolerated and noninvasive; this is essential for general acceptability of the test.
Results
The TB Breathalyzer has been field-tested with over 900 patients in India and Ethiopia; the most recent trials of 427 patients in Ethiopia demonstrated sensitivity and specificity >95%.2
Benchmarking the TB Breathalyzer took some doing because there are no predicate devices based on breath samples. Ultimately, clinical examinations by medics and checking x-rays of patients proved the most reliable ways to evaluate the system. Results of testing the TB Breathalyzer correlate with patient testing using these methods, as well as sputum smear microscopy—the method still regarded as the "standard" for diagnosing TB (but which takes weeks to prepare cultures and also requires lab facilities). The Breathalyzer test detects actively infectious TB bacilli 'free' to be coughed out and the TB results are unaffected by other conditions such as HIV, upper respiratory tract infections (URTI), and cancer. The limit of detection is estimated at ~25–75 TB bacilli. The test can diagnose TB in patients who do not produce sputum, which typically can be up to 50% of patients sampled.
An exciting feature of the system is that it has been found to be capable of detecting TB in the very early stages, when the disease is initially forming and the TB bacilli are lodged and multiplying in the upper respiratory tract, before they cause lung damage that can be detected in an x-ray or can get into the sputum.
Of course, other technologies are being developed to address the pressing need. One that has been considered promising is PCR technology, though like the current standard, the Mantoux tuberculin skin test, it takes hours not minutes and it is more complicated to administer.
REFERENCES
1. See http://bit.ly/1m0bs78.
2. R. McNerney et al., BMC Infect. Dis., 10, 161 (2010); http://dx.doi.org/10.1186/1471-2334-10-161.
3. Patents: Rapid Biosensor Systems Ltd. 'Biological Measurement System' (PCT WO 02/084266 A2) and 'Bioassay and Peptides for use therein' (WO2007/072063).
Dennis Camilleri is CEO of Rapid Biosensor Systems Ltd., www.rapidbiosensor.com.
Bio-optical processing
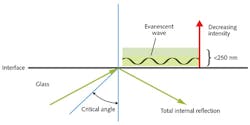
The TB Breathalyzer works on the principle of evanescent waves, which result when light is refracted as it travels from one medium into another with a different refractive index. The critical angle is reached when the angle of the incident light gives rise to refraction at 90 degrees to the perpendicular. When the incident angle is greater than the critical angle, total internal reflection results: A small portion of the reflected light penetrates through the interface, creating a very thin electromagnetic field (<200–250 nm) adjacent to and propagating along the interface. This is the evanescent wave.
Evanescent means "tending to vanish" and this is a good description because the intensity decays exponentially (rather than sinusoidally) with distance from the interface at which it is formed. In evanescent wave immunobiosensors, antibodies are attached to a glass surface and the evanescent light propagating through the near surface region excites any fluorescence that is immobilized on the surface.