ORGANIC PHOTOVOLTAICS: Transition metal oxides increase organic solar-cell power conversion
MARK T. GREINER, LILY CHAI, and ZHENG-HONG LU
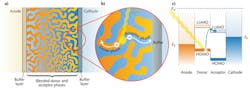
Organic photovoltaics offer an inexpensive alternative to traditional inorganic solar cells. They also allow for a flexible form factor that makes possible novel portable power applications and unique fabrication processes, such as roll-to-roll printing (see Fig. 1). These factors may provide the avenue to popularize renewable energy in the consumer electronics market.
Although organic photovoltaics (OPVs) have cost and manufacturing advantages over inorganic PVs, they are hindered by low power conversion efficiency (PCE). However, recent progress in OPV research has led to substantial PCE gains that are making OPVs commercially attractive. These improvements are largely due to the realization that interface engineering is essential for achieving high-efficiency OPVs.
Organic PV operating principles
In a solar cell, light absorption generates mobile charge carriers that are collected by two electrodes to generate a voltage. In OPVs, organic molecules absorb the light. Typically OPVs use the bulk heterojunction design.
In a bulk heterojunction, a blend of donor and acceptor molecules form separate phases that percolate throughout the device (see Fig. 2). The blended organic phases are sandwiched between two electrodes, an anode and a cathode.During light absorption, an electron moves from the highest occupied molecular orbital (HOMO) of a donor molecule into the lowest unoccupied molecular orbital (LUMO) of an acceptor molecule, leaving behind a positively charged "hole" on the donor molecule. The electron and hole move in opposite directions toward the two electrodes. Electrons are collected by the cathode and holes by the anode.
The interface between an organic phase and an electrode is critical for efficient charge collection and attaining high PCEs.1 As charges cross an interface, a voltage drop can occur, resulting in a decrease in PCE. Furthermore, if an electrode is not charge selective then leakage currents will occur, further decreasing the PCE.2 Cathodes should only collect electrons and anodes only holes. Voltage drops and leakage currents can be minimized by incorporating an appropriate buffer layer between the electrodes and the organic phases.
Transition metal oxides have proven to be very useful electrode buffer layer materials.3 They allow interfacial energy-level alignment to be tuned to maximize cell voltage and they have rectifying properties that decrease leakage currents. Transition metal oxides exhibit a wide range of electronic properties that can be tuned through their chemical treatments, which makes them very versatile materials.4 Some oxides are suitable as cathode buffer layers and others as anode buffer layers.
Power conversion efficiency
Defined as the ratio of outgoing electrical power to the incoming solar power, PCE is the product of three parameters: open circuit voltage (VOC), short circuit current (JSC), and fill factor (FF).5
The VOC is the maximum voltage that a solar cell can generate, JSC is the maximum current it can generate, and FF is a parameter that describes the maximum power it can generate.
All three factors—VOC, JSC, and FF—depend heavily on electrode/organic interfaces. With the incorporation of a metal oxide buffer layer at electrode/organic interfaces, these three factors can be optimized to boost an OPV's performance.
Energy level alignment and VOC
The maximum VOC that an organic solar cell can achieve depends on the offset between a donor molecule's HOMO and an acceptor molecule's LUMO.6 However, the maximum VOC can only be achieved by appropriately tuning the energy-level alignment at the electrode/organic interfaces.
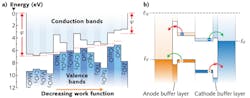
Energy-level alignment refers to the offset between the HOMO or LUMO levels and an electrode's Fermi level. These offsets are labeled as ΔHOMO and ΔLUMO, respectively (see Fig. 3). In order to have a zero voltage drop at an interface, the HOMO (LUMO) level should be in perfect alignment with the anode (cathode) Fermi level. At the anode interface, ΔHOMO should be zero, and at the cathode interface, ΔLUMO should also be zero.It was recently shown that metal oxides exhibit a broad spectrum of energy-level aligning capabilities.7 When an electrode's Fermi level is below a molecule's HOMO level, the minimum ΔHOMO is achieved, and when an electrode's Fermi level is above a molecule's LUMO level, the minimum ΔLUMO is achieved.
An electrode's Fermi level is parameterized by its work function φ. Work function represents the energy lost (gained) when removing (adding) an electron from a material. The maximum VOC can be achieved when the anode's work function is greater than the donor molecule's ionization energy and the cathode's work function is less than the acceptor molecule's electron affinity. Consequently, the maximum VOC is proportional to the energy offset between the donor's HOMO level and the acceptor's LUMO level. If HOMO and LUMO levels are not aligned with the respective electrode Fermi levels, then the VOC will be less than the ideal value.
Transition metal oxides can be used to tune electrode work functions in order to maximize VOC. They are capable of a wide range of work functions. For example, oxides of titanium, zinc, tantalum, and zirconium—such as TiO2, ZnO, Ta2O5, and ZrO2—have low work functions and can be used as cathode buffer layers. Other oxides of molybdenum, nickel, copper, and vanadium—MoO3, NiO, CuO, and V2O5—have high work functions and can be used as anode buffer layers.
Metal oxides have the advantage over metal electrodes because oxides can achieve much higher work functions than metals. The highest known metal work function is approximately 5.3 eV for platinum (Pt), while the highest known oxide work function is approximately 7.0 eV for vanadium oxide (V2O5). Furthermore, the high-work-function metals are expensive noble metals—such as gold (Au) and Pt—while the high-work-function oxides are low-cost minerals such as MoO3 and V2O5.
Metal oxides also have an advantage over metals for low-work-function electrodes. The metals that have low work functions, such as calcium (Ca) and magnesium (Mg), tend to be very reactive, and when in contact with organic molecules they will break the molecule's chemical bonds. On the contrary, low-work-function oxides are chemically inert, such as zirconium oxide (ZrO2) and titanium oxide (TiO2).
Leakage current and JSC and FF
Both JSC and FF are affected by leakage currents. In a photovoltaic device, if an electrode collects both holes and electrons, the result is a decrease in net current and a consequent decrease in JSC and FF.
Metallic electrodes are not charge selective; oxides can be if they have the right electronic band structure. Transition metal oxides can have a range of electronic structures—from p-type semiconductors to n-type semiconductors (see Fig. 4). Wide-bandgap p-type oxides with high work functions are ideal anode buffer layers because they allow holes to pass but not electrons. Wide-bandgap n-type oxides with low work functions are ideal for cathodes because they allow electrons to pass, but not holes.The highest-reported PCE for OPVs is approximately 8%. Energy aligning and charge-selective electrode buffer layers are essential for such high efficiencies. Transition metal oxides are versatile buffer layers because of their energy-aligning and charge selective properties, and they will continue to play a role in improving OPV PCE values.
REFERENCES
1. W.Z. Cai et al., Solar Energy Mat. and Solar Cells, 94, 2 (2010).
2. E.L. Ratcliff et al., J. Phys. Chem. Lett., 2, 11 (2011).
3. T. Gershon, Mat. Sci. and Technol., 27, 9 (2011).
4. M.T. Greiner et al., Nat. Mat., 11, 1 (2012).
5. B. Kippelen and J.L. Bredas, Energy & Environ. Sci., 2, 3 (2009).
6. R. Po et al., Energy & Environ. Sci., 4, 2 (2011).
7. C. Tengstedt et al., Appl. Phys. Lett., 88, 5, 053502 (2006).
Mark T. Greiner and Lily Chai are PhD researchers and Zheng-Hong Lu is professor and Canada Research Chair in Organic Optoelectronics, Tier 1, in the Department of Materials Science and Engineering at the University of Toronto, ON, Canada; e-mail: [email protected]; www.utoronto.ca.