BHARAT R. ACHARYA
Nematic liquid crystal (NLC) is the electrically responsive fluid behind most flat-panel displays in which the orientation of the NLC is manipulated using an external electrical field to display information. In recent years, there have been significant advances in unconventional applications of NLCs in photonics, sensing, and diagnostics. For sensing applications, the alignment of these NLCs, supported on a chemically functionalized surface, can be disrupted in the presence of a target analyte. The sensitivity, selectivity, and reversibility of the sensor for a variety of analytes can be tuned to address many industrial and environmental sensing applications.
Liquid-crystal reorientation by chemical vapors
Liquid crystals (LCs) typically consist of anisotropic molecules that possess long-range orientational correlation (crystalline-like ordering) but either lack or have short positional correlations (liquid-like ordering). Among various liquid crystalline phases, the molecules in NLC materials have a tendency to align parallel to each other without any positional ordering. The crystalline-like ordering permits the orientation of NLCs at an LC-substrate interface to propagate through the bulk of the film supported on a solid substrate. The liquid-like ordering enables extreme sensitivity to external stimuli such as electric, magnetic, or surface fields. These unique characteristics of NLCs make them attractive for use in electro-optic display devices. In such devices, a thin film of NLC is supported between two electrodes and the orientation of the NLC is manipulated by applying an electric field to modulate the light propagating through the NLC film to display information.
The orientation of NLCs at a LC-substrate interface is extremely sensitive to the molecular structure of that interface and is dictated by the orientation of the first layer of NLC molecules in contact with the surface. This property of LCs has been used to achieve a uniform alignment of a thin (tens of microns) film of NLCs on rubbed polymer films in the LCD display industry.1 By designing substrates with select surface properties, this principle has also been exploited to detect and report binding of biological entities and chemical molecules at LC-aqueous and LC-substrate interfaces.2, 3 These LC sensors form the basis of low-power-consumption, low-cost, lightweight, and easy-to-use detectors suitable for numerous applications such as the detection of DMMP, an organophosphonate compound and simulant of the nerve agent Sarin.
Sensor fabrication
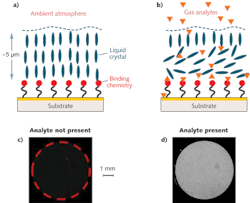
An NLC-based sensor is composed of a thin film of NLC supported on a surface that is chemically functionalized for a specific target (see Fig. 1). The NLC molecules adopt a predefined orientation when supported on a surface functionalized with binding chemistry. The binding chemistry is selected to have a higher affinity for the targeted analytes than for the functional groups on the NLC. When a sensor is exposed to the test environment, targeted analytes diffuse through a thin (micrometers depth) film of NLC to bind to the chemistry on the surface. The analytes displace the NLCs at the interface from their interaction with the surface chemistry and cause the NLC molecules to change their orientation. This re-orientation propagates through the bulk of the film and can be detected by exploiting the anisotropic optical properties of LCs–that is, by viewing the sensor between crossed polarizing filters.
Some NLC materials, when supported between two surfaces treated with select transition metal complexes, assume an alignment perpendicular to the surface.4 Using surfaces functionalized with transition metal complexes, the selective binding of organophosphonate compounds, such as di-isopropyl methylphosphonate (DIMP), has been demonstrated using infrared (IR) spectroscopy.5 Exploiting the sensitivity of the LC to molecular changes at the LC-substrate interface, the binding of chemical vapors on a copper (Cu2+) functionalized surface was detected and reported using NLCs.3 Using this principle, Platypus Technologies has developed NLC-based DMMP sensors that are stable, reproducible, and immune to potentially interfering compounds (PICs).
The NLC-based DMMP sensors are fabricated on a glass substrate with a 5 mm diameter active area comprising polymer micropillars that are 5 μm in height, 10 μm in diameter, and spaced (center-to-center) 20 μm apart. The substrates are coated with a 2 nm titanium adhesion layer followed by a 10 nm thick gold film. A self-assembled monolayer of mercaptoundecanoic acid is formed on the gold-coated substrate and then functionalized with aluminum ions (Al3+) by spin-coating an aluminum perchlorate solution. Finally, a thin film of LC E7 containing the nitrile (-CN) group is overlaid on the treated substrate. The micropillars stabilize and maintain a uniform NLC film (approximately 5 μm thick) across the active area.
The sensors are exposed inside a customized exposure chamber flanked by two crossed polarizers between a diffuse light box and a charge-coupled device (CCD) camera. The setup is placed inside an environmental chamber maintained at a constant temperature and a customized gas delivery system [mass flow controllers, zero air (dry air free of NOX, SO2, NO2, O3, SO2, and H2S) sources, solenoid valve, and standard DMMP gas cylinders] is used to generate the desired DMMP concentration. A humidified gas stream is generated by bubbling zero air through water and mixing it, at appropriate ratios, with DMMP gas. The sensors are exposed at a flow rate of 1 liter/min to different concentrations of DMMP and PICs either using standard gas cylinders or by bubbling nitrogen through respective liquids to generate vapors. The optical response (that is, amount of white light) from the sensor is determined by calculating the mean grayscale intensity of the digital images captured by the CCD camera.
Sensitive and selective detection
The interaction between the -CN group of the NLC and the Al3+ on the sensor surface causes the NLC molecules at the interface to align perpendicular to the surface. This alignment propagates through the entire film and no light is transmitted through the sensor placed between crossed polarizers. When the sensor is exposed to DMMP, the molecules rapidly diffuse through the NLC film and bind to the Al3+ ions at the interface. Since the Al3+ ions coordinate more strongly with the phosphoryl group of DMMP than to the -CN group of the NLC, the DMMP displaces the NLC at the interface. Once the surface coverage density of the coordinated DMMP molecules exceeds a threshold, the NLC film undergoes an orientational transition and allows transmission of light through the sensor.
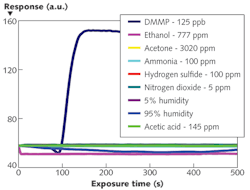
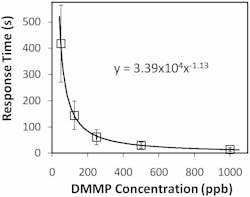
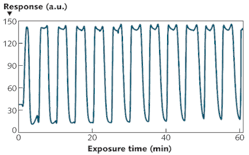
The NLC-based sensor yields a measurable response to 125 parts per billion (ppb) DMMP in less than 2 min (see Fig. 2). The response rapidly increases, then saturates and remains steady while DMMP is present. The data also shows that the sensors are not responsive to other PICs tested at concentrations at least 40X higher than that of DMMP. This selective detection of DMMP against tested PICs results from the higher binding affinity of DMMP to Al3+ in comparison with other chemicals. Moreover, the sensor does not respond to humidity–a distinguishing attribute of the NLC-based sensors compared with existing sensors based on other technologies. This imperviousness to humidity can be attributed to the diminished binding affinity of water molecules to Al3+ ions at the interface in the presence of -CN functional groups of NLC.In addition to this robust, small, and inexpensive DMMP sensor, we have demonstrated the versatility of our NLC-based platform by using different binding chemistries such as metal complexes, thiols, and polymers to detect various gases including oxides of nitrogen, ozone, and hydrogen sulfide that are of interest in numerous biomedical, environmental, industrial hygiene, and homeland security applications.
ACKNOWLEDGMENTS
The author would like to acknowledge contributions from the Platypus Technologies team including Darrin Most, Avijit Sen, Heidi VanTreeck, Bart Grinwald, Kurt Kupcho, and Michael Bonds; and financial support from the US Department of Defense, the National Institutes of Health (NIH), and the National Institute of Environmental Health Sciences (NIEHS).
REFERENCES
1 . B. Bahadur, Liquid Crystals: Applications and Uses, 3, World Scientific, Singapore (1992).
2. V.K. Gupta, T.B. Dubrovsky, and N.L. Abbott, Science, 279, 2077-2080 (1998).
3. R. Shah and N.L. Abbott, Science, 293, 1296-1299 (2001).
4. S. Furunza et al., Liquid Crystals,16, 277-285 (1994).
5. R.M. Crooks et al., Faraday Discussions, 107, 285-305 (1997).
Bharat R. Acharya is director of sensor development at Platypus Technologies LLC, 5520 Nobel Dr., Suite 100, Madison, WI 53711; e-mail: [email protected]; www.platypustech.com.