SINGLE-PHOTON DETECTORS: Fluorescence microscopy benefits from advances in single-photon detectors
RICHARD K. P. BENNINGER and DAVID W. PISTON
The study of biomolecular interactions and dynamics in live cells, tissue, and whole organisms (intravital imaging) has greatly increased over the past decade. Intravital microscopy is now widely used in neuroscience, immunology, and developmental biology to study the dynamics of specific proteins, lipids, or whole cells in live organisms. In part, this increased use has been due to the development and refinement of genetically encodable green fluorescent protein; however, the development of high-speed ultrasensitive confocal microscopes and two-photon microscopes has also played a significant role.
One of the central components of the laser-scanning confocal or two-photon microscope is the photon detector. The sensitivity of photon detection effectively limits the speed and resolution at which specific biological functions can be studied. Generally, the level of fluorescent signal emitted from a biological sample is low, often several orders of magnitude less than the excitation intensity. Furthermore, low excitation light levels are necessary to avoid perturbing effects of photobleaching and phototoxicity. As a consequence, the development of high-sensitivity photon detectors has been central to recent advances in fluorescent microscopy.
Detection options
Modern photon detectors need to have high photon-detection efficiency, fast response and readout, low noise level, low background level and, most important, must be extremely reliable. Charge-coupled devices (CCDs) are generally used in wide-field microscopes, while the most widespread photon-detecting devices in laser-scanning microscopes are photomultiplier tubes (PMTs).
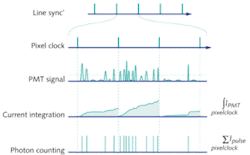
In almost all commercial microscopes, PMTs are run in “current integration” mode in which the output current is integrated over the time the laser is scanned over a single pixel of the image (the pixel dwell time). The integrated current is then converted into a voltage and digitized to an 8- or 16-bit digital number (DN) that is proportional to the number of photons collected from the pixel of the sample (see Fig. 1).
This mode of detection is suitable for moderate to high levels of photon flux. However, at low signal levels, significant noise is introduced by the PMT amplification and digitization. The high gain required for a low photon flux leads to large multiplicative noise, creating a wide variation in the magnitude of the resultant current pulse, as well as significant after-pulsing. Thermally generated electrons can also be produced in the photocathode and dynode chain leading to an elevated dark signal with an associated noise.
Single-photon counting (SPC) or pulse counting is another form of detection that can be used in photon-detection devices such as PMTs. Photon-counting PMTs are generally cooled to reduce the number of thermally generated dark electron events. High-bandwidth electronics and a high gain give a tightly distributed current pulse that is well separated from background current. Each current pulse can then be discriminated and counted to give the absolute number of photons detected and eliminate the additional dark signal and noise that is introduced in current integration mode. The noise in this signal is effectively shot-noise limited and can be described by well-defined Poisson noise statistics.
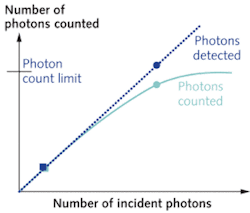
Despite the obvious advantages of photon counting, its use in fluorescence microscopy has been limited. The main limitation is the rate at which photons can be counted in the PMT module and detection electronics. During the time taken to generate, discriminate, and count a single current pulse, other incident photons will not be counted. Thus, the maximum count rate is related to the time taken to count a single photon (the dead time). The maximum count rate is approximately the inverse of the dead time; however because photons arrive randomly in time, even at a lower photon flux two or more photons can still be incident within the dead time. The linearity of detection, where the number of photons incident is proportional to the number of photons counted, occurs for less than 10% of the maximum count rate. Traditionally, this limit has been below 1 MHz. For a typical biological image acquisition time of one second and 512 × 512 pixels, this would limit the maximum counts per pixel to approximately four. But within the past few years, novel technology that improves upon this approach is gaining increased attention for microscopy applications.
Improving the photon-counting method
Recently developed photon-counting PMT modules now have maximum count rates of tens of megahertz. For example, the H7421-50 module from Hamamatsu (Hamamatsu City, Japan) has a pulse-pair resolution of 20 ns, for a maximum count rate of 50 MHz. This is far above the level of photons that would be expected from even a brightly fluorescent biological sample.
Another development has been field-programmable gate arrays (FPGAs), which allow flexible logic to be programmed into a chip to provide the high-speed operations required to discriminate, count, and read out current pulses as the laser excitation is scanned over a single pixel of the sample (with a duration of a few microseconds). These FPGA devices also have the ability to handle transistor-transistor logic (TTL) or emitter-coupled logic (ECL) at tens of megahertz, meaning that the building blocks are now available to develop fast photon-counting microscope detectors that can handle up to several hundred photons per pixel for typical image sizes and speeds.
Our group at Vanderbilt University recently described the implementation of a simple photon-counting device to provide enhanced detector sensitivity in a two-photon microscope. Using a Hamamatsu H7421 series photon-counting PMT module, we demonstrated quantitatively that at low levels of fluorescence the home-built photon-counting detector provides a higher signal-to-noise level than the commercial microscope PMT running in current integration mode (see Fig. 2).1 For a sample microscope image, a signal-to-noise comparison was made for varying concentrations of the endogenous fluorescent enzyme cofactor NADH. Imaging this endogenous fluorescence is routinely used as an assay of cellular metabolic activity and thus we demonstrate that enhanced sensitivity can be obtained in biologically relevant applications. Our analysis also showed that the signal remains shot-noise limited—the fundamental noise limit in any fluorescence imaging application. Our detector would be improved further by incorporating the precision optical design and components present in the commercial microscope PMT detector.
The power of applying FPGA logic to photon-counting detection has been further demonstrated in a study from Peter So’s laboratory at the Massachusetts Institute of Technology (MIT).2 A customized photon-counting card allows discrimination and counting of 16 parallel channels, each with an approximate 30 MHz count-rate maximum, linear up to 1 MHz count rate. A multi-anode PMT unit can then be incorporated with this photon-counting card to perform hyperspectral imaging where the fluorescence emission is dispersed across the multi-anode PMT unit.
Some newer microscope systems are starting to default to a multi-anode photon-detection system. For example, the Carl Zeiss (Oberkochen, Germany) LSM710 confocal microscope uses a multichannel PMT hyperspectral detector (the Quasar detector), where the fluorescence from the sample is distributed between multiple PMT detection units, according to the emission wavelength. Multiple parallel 30 MHz discrimination and counting systems would be particularly attractive for this sort of system and should further improve the detection sensitivity.
The increased use of live-cell imaging and intravital microscopy over the past few years provides a strong motivation for continually improving the sensitivity of photon detectors in fluorescent microscopes.3 Evidence suggests that the technology is now available to incorporate photon-counting detection in commercial microscope systems. We expect that microscope manufacturers will start to incorporate this technology in the next generation of microscopes, and that this in turn will open up new frontiers in live-cell microscopy.
ACKNOWLEDGMENT
The authors wish to acknowledge funding from NIH grants R01-DK53434 and P20-GM72048 and the Department of Defense Medical Free-Electron Laser Program.
REFERENCES
- R.K.P. Benninger et al., Optics Lett. 33(24) p. 2895 (Dec. 15, 2008).
- C. Buehler et al., J. Fluorescence 15, p. 41 (2005).
- R.K.P. Benninger et al., Rev. Physiology, Bio chemistry and Pharmacology 160, p. 71 (April 2008).
Richard K. P. Benninger is a research fellow and David W. Piston is a professor in the Department of Molecular Physiology and Biophysics at Vanderbilt University Medical Center, 1211 Medical Center drive, Nashville, TN 37232; e-mail: [email protected]; www.vanderbilt.edu.