Full-field OCT is an en face (transverse), broadband interferometric approach to optical coherence tomography (OCT) that’s been in use for life-science research for more than a decade.1—3 The technique offers many advantages, including fast tissue imaging at the cellular level, which is why at ESPCI (Ecole Superieure Physique Chimie Industrielles; Paris, France) we call it “Cell OCT.” Recently, full-field Cell OCT proved able to virtually slice ablated breast tumors and lymph nodes with 1 µm isotropic resolution, and to produce images that compare favorably with the histology sections of the same tissues. These results confirm the potential of this approach for histopathology, which implies reduced trauma for patients and lower costs for health-care providers.
The approach and the system
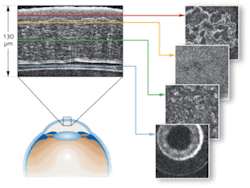
Unlike most OCT approaches, which produce axially oriented images, full-field Cell OCT acquires tomographic images in the en face or transverse orientation.4, 5 It uses an interferometer to move the focal plane at different depths below the surface to produce 3-D tomographs. En face capture enables high lateral resolution (typically approximately 1 µm) using medium- or large-aperture microscope objectives. In comparison, time-domain, spectral, Fourier-domain and swept-source OCT require low-numerical-aperture optics–with a large depth of field–that limit lateral resolution to between 5 and 40 µm.
With all OCT approaches, axial sectioning is linked to the coherence length of the light source. Sophisticated and expensive femtosecond lasers have been used in laboratory setups to image sections in the micrometer range, whereas commercial OCT systems use spatially coherent superluminescent diodes typically able to section at 10 µm. Our approach takes advantage of broadband, spatially incoherent sources such as thermal tungsten filament lamps or Xenon arcs to achieve submicron slicing.
Full-field Cell OCT relies on low-coherence interference microscopy. The experimental arrangement is generally based on a bulk Michelson interferometer with identical microscope objectives in both arms–a configuration referred to as the Linnik interferometer. A spatially incoherent source illuminates the entire field of the microscope objective. The low temporal coherence of the source means that interference occurs only when the optical path lengths of the two interferometer arms are within 1 µm of each other. Indeed, the interference fringes are contained within a narrow envelope whose width is equal to the coherence length of the source divided by 2n (where n is the refractive index of the sample).
When a biological sample is placed under the microscope objective, the light reflected by the reference mirror interferes with the light reflected or backscattered by the sample contained in a limited volume. This volume is a slice orthogonal to the objective axis, located within the sample at a depth defined by an optical path length difference of zero. The thickness of the slice is determined by the width of the fringe envelope. Extraction of the signal yields an en face tomographic image.
The system extracts the signal from the large background of incoherent backscattered light through phase shifting. Most of the time, a CCD camera records two interferometric images successively, with phase being changed by p in the interferometer between them. Phase shifting is accomplished by displacement of the reference mirror with a piezoelectric translation stage. The tomograph is calculated by combining the interferometric images.
Applications and results
Researchers at the ESPCI have used Cell OCT for more than 10 years for such biomedical applications as in vitro and in vivo anterior eye examination (see Fig. 1), studying mouse embryo development, and understanding programmed cellular death. But it had never been developed as a clinical tool. Last year, LLTech (Lille, France), a company formed to provide cellular-resolution imaging devices to enable noninvasive, nondestructive optical biopsies, proposed to Dr. Brigitte Sigal in the Senology Department of the Curie Institute (Paris, France), that she and her colleagues explore Cell OCT. The Institute is a private foundation providing cancer research, diagnosis, and treatment services, and LLTech, which is now working to commercialize Cell OCT, thought the approach could complement the pathologists’ work on breast-cancer diagnostics.
We have worked closely with the Curie Institute comparing the virtual slices from the Cell OCT, obtained without any preparation, with the physical (histological) slices. Standard histology requires significant manual preparation of samples before they can be viewed under a light microscope–a sample must be frozen or embedded after dehydration and infiltration with fixative, stained to provide contrast, and then sectioned. Because sections are realigned after they have been cut, reconstruction can be warped. The technique cannot be applied in vivo, and ex vivo samples are vulnerable to damage.
In contrast, we demonstrated that Cell OCT can produce images of comparable quality–its isotropic resolution, approximately 1 µm, is close to that of standard histology–but quickly and noninvasively, without staining. The technique is able to image slices in the sample, making possible 3-D reconstruction of the sample morphology. And it can be applied to the whole sample, which is a considerable advantage over traditional methods (see Fig. 2).Our work with breast tissue confirms the potential of our new tool for virtual histopathology.
Looking forward, we plan to implement a setup in the Curie Institute’s operating room to enable fast diagnostics on the tumor margins and lymph nodes. A 10-minute analysis should improve the quality of the “macroscopic” observation diagnostic. What’s more, it should enable reduction of the reoperation rate (that is, the need for patients to return for further surgery), which ranges between 12% and 40%. Therefore, in addition to reducing pain for the patient, this approach should also reduce health-care costs. LLTech is planning to commercialize full-field Cell OCT in 2010.
REFERENCES
- J.G. Fujimoto et al., Nature Med. 1, p. 970 (1995).
- A.F. Fercher, J. Biomed. Opt. 1, p. 157 (1996).
- G.J. Tearney et al, Opt. Lett. 21, p. 1408 (1996).
- A. Dubois et al., Appl. Opt. 41, p. 805 (2002).
- L. Vabre et al., Opt. Lett. 27, p. 530 (2002).
- S. Labiau et al., BiOS (a part of Photonics West), 7169-30, Session 5 (2009).