RAMAN SPECTROSCOPY: Spatial offset broadens applications for Raman spectroscopy
CHARLOTTE ELIASSON AND PAVEL MATOUSEK
In many analytical applications it is necessary to ascertain the chemical composition of deep layers in diffusely scattering (turbid) samples. Examples include the noninvasive probing of living tissue in disease diagnosis or the noninvasive probing of pharmaceutical tablets and capsules in quality control. Raman spectroscopy holds particular promise in this area because of its high chemical specificity, compatibility with water-containing samples, and ease and speed of implementation.
The Raman effect involves the inelastic scattering of photons from molecules via interactions with the vibrational modes of the analyte molecule.1 In this process, a photon typically transfers a fraction of its energy to a vibrational mode within the molecule, and consequently its wavelength is red-shifted. The degree of the shift corresponds to the amount of energy taken up by the molecule. Because the vibrational modes are quantized and their energy spacing is molecule-specific, the molecular identity can be determined from the pattern of the observed shifts. The general applicability of this technique is limited to samples that do not exhibit strong fluorescence emission in the Raman spectral range, as strong emissions can easily swamp the relatively weak Raman signals. This problem can often be eliminated or minimized, however, using near-IR excitation away from the electronic absorption bands of most fluorescing species.
To date, the Raman method has been used predominantly in the backscattering collection geometry (see Fig. 1, left). In this mode, it is, however, only capable of probing shallow depths of turbid media where the medium still appears semitransparent.1 For example, in living tissue the accessible depths are typically only several hundred microns. The deeper layers remain inaccessible because Raman or interfering fluorescence signals originating from the surface layer mask the much weaker subsurface Raman spectra.
A substantial extension of the penetration depth was recently accomplished through the development of spatially offset Raman spectroscopy (SORS; see Fig. 1, right).2 The method utilizes the diffuse component of light, similar to NIR absorption tomography. The development of this method stems from earlier studies into the Raman migration process using an ultrafast Raman Kerr gate.3-6 The SORS method is based on the collection of Raman spectra from spatially offset regions away from the point of illumination on the sample surface. The laterally offset Raman spectra contain different relative contributions from sample layers located at different depths. This difference is brought about by a wider lateral diffusion of photons emerging from greater depths.
The offset spectra can either contain acceptably low Raman contribution from the surface layer, or if a considerable signal is still present then this can be further removed using a scaled subtraction of spectra obtained at different spatial offsets. Alternatively, the separation of Raman signals originating from different layers in the sample can be accomplished using multivariate data analysis applied to a set of spatially offset Raman spectra. The SORS approach is also capable of effectively suppressing the interfering fluorescence if it originates from the surface layer of the probed medium.
Since the development of the SORS concept, the technique has been used in numerous applications, including the demonstration of Raman tomography in turbid media, the noninvasive Raman spectroscopy of bones, and in pharmaceutical and security applications.7-11
Detecting counterfeit drugs
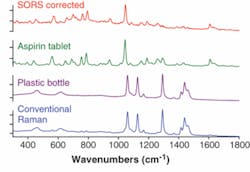
There is a strong need to screen the content of pharmaceutical products throughout the supply chain, stemming from the infiltration of the market by counterfeit products.12, 13 Such fake drugs can present a serious health risk and in extreme situations—for example, in the case of counterfeit antimalarial tablets—a serious life threat. Although the Raman technique in its conventional form already is used in this area, its effectiveness varies depending on the color and thickness of the packaging. Generally, the technique can cope well with lightly colored capsules and tablets held in blister packs. However, darkly colored capsules or thicker packaging (such as white plastic bottles) can yield excessive amounts of Raman and/or fluorescence emission that can interfere with the much weaker Raman signals of the active ingredients inside-thus reducing the sensitivity of the technique.
Spatially offset Raman spectroscopy has shown a substantially higher degree of sensitivity in this application area than conventional Raman spectroscopy, primarily because of its ability to effectively suppress Raman and fluorescence contributions from the packaging. It has enabled noninvasive probing of pharmaceutical products, including drugs contained within opaque plastic bottles. In noninvasive probing of white plastic bottles containing aspirin tablets, the conventional Raman spectrum is heavily contaminated with intense interfering Raman signals from the container, masking the Raman signature of aspirin inside (see Fig. 2). In contrast, the SORS approach, after the scaled subtraction of two SORS spectra measured at different spatial offsets (0 and about 10 mm), yields a clean Raman spectrum of aspirin within the bottle. Comparative experiments were performed at 830 nm, with an acquisition time of 1 s and a laser power of 250 mW.Security screening of envelopes
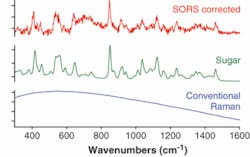
The current heightened terrorist threat calls for more-robust and effective security screening methods with high chemical specificity. Recently we reported the use of SORS in probing powders held in envelopes, an application suitable for deployment in mail sorting centers. We demonstrated one example of screening of a brown envelope (0.14 mm thick) for the presence of a harmful substance or illicit drug in powder form, which in our case was represented by sugar (see Fig. 3). The conventional backscattering Raman measurement yielded an overwhelming fluorescence background that swamped the much weaker Raman spectrum of sugar. The SORS spectrum, however, clearly revealed the Raman components of sugar held within the envelope. The experiments were performed at 830 nm and the acquisition time was 1 s with a laser power of 250 mW.The development of the SORS method for deep subsurface probing of opaque materials heralds the arrival of new analytical applications, from quality control to disease diagnosis and security screening. These developments come at a time when Raman spectroscopy is completing its transformation from laboratory technique to practical analytical tool. The SORS capability can be readily incorporated into commercially available robust handheld Raman instruments. Consequently many of these new applications may rapidly find a way into our daily lives.
REFERENCES
1. Analytical Applications of Raman Spectroscopy; Edt. M. J. Pelletier, Blackwell Science, Oxford, 1999.
2. P. Matousek et al., Appl. Spectroscopy 59, 393 (2005).
3. P. Matousek, M. Towrie, A. Stanley, A. W. Parker, Appl. Spectroscopy 53, 1485 (1999).
4. N. Everall, Appl. Spectroscopy 55, 1701 (2001).
5. P. Matousek, N. Everall, M. Towrie, A. W. Parker, Appl. Spectroscopy 59, 200, (2005).
6. E. R. C. Draper et al., J. Bone and Mineral Research 20, 1968 (2005).
7. M.V. Schulmerich, W.F. Finney, R. A. Fredricks, M. D. Morris, Appl. Spectroscopy 60, 109 (2006).
8. M. V. Schulmerich, et al., J. Biomedical Optics 11, 060502 (2006).
9. P. Matousek et al., Appl. Spectroscopy 60, 758 (2006).
10. C. Eliasson, P. Matousek, Analytical Chemistry 79, 1696 (2007).
11. P. Matousek, Appl. Spectroscopy 60, 1341 (2006).
12. P. N. Newton et al., Lancet Infectious Diseases 6, 602 (2006).
13. M. R. Witkowski, American Pharmaceutical Rev. 8, 56 (2005).
CHARLOTTE ELIASSON is an instrument scientist and PAVEL MATOUSEK is a senior scientist at the Central Laser Facility, CCLRC Rutherford Appleton Laboratory, Didcot, Oxfordshire, OX11 0QX, England; e-mail: [email protected]; www.cclrc.ac.uk.