Stochastic scanning multiphoton multifocal microscopy combines a diffractive-optic-generated array of spots with random-access scanning to achieve rapid, high-resolution microscopy without artifacts.
JUSTIN E. JURELLER, HEE Y. KIM, AND NORBERT F. SCHERER
Fluorescence microscopy is a workhorse of biophysics and cell biology.1 Imaging of fluorescence from endogenous or exogenous fluorophores is used to reveal structural localization, binding, expression, or conformational state of proteins or other objects of interest at submicron length scales. However, live biological systems such as cells are dynamic, with processes occurring on all time and length scales. Rapid high-resolution imaging or correlation measurements are needed to resolve processes (for example, signaling or intracellular transport of vesicles and proteins) that occur on the order of milliseconds or faster.2
The need for such dynamic imaging has driven the development of high-speed multiphoton and confocal-scanning fluorescence microscopies.3 Current challenges include improving spatial resolution “beyond the diffraction limit” and increasing imaging speed beyond video rates.4 These dual goals allow seeing finer detail while understanding cellular processes on native timescales. Unfortunately, extending traditional scanning microscopies to higher speeds introduces spatiotemporal artifacts. This means the complexity of instruments with the required performance to capture fast dynamics without artifacts has grown concurrently. The high cost and “black-box” design of commercial instruments create a large barrier that impedes widespread adoption and user development of new dynamic imaging techniques. To address these issues, we have developed stochastic scanning multiphoton multifocal microscopy (SS-MMM) as a simple, yet high-performance, alternative.5
The conventional approach
A conventional scanning fluorescence microscope creates an image by progressively shifting a laser-excitation spot across a sample located at the focal plane of a high-magnification objective. The fluorescence generated by fluorophores is either descanned and imaged onto a single-element photomultiplier tube or avalanche photodiode (as in the one-photon case with a confocal pinhole), or imaged to a plane of an array or wide-area detector (as in the case of two-photon microscopy). Each approach requires tight synchronization of detection electronics with a laser beam scanner.
Typically, x and y galvanometer mirrors driven with raster-scan waveforms are used to scan the laser spot and serially build up a single frame. The mechanical properties of galvanometers (for example, bandwidth and phase lags) often limit maximum overall frame rates to a few hertz. Even then, complications such as beam-blanking at the edge of the scanning pattern are required to ensure an even and uniform excitation dwell time at each pixel position. Such effects are exacerbated at higher speeds and adversely impact duty-cycle efficiency, cost, and complexity. Resonant and Nipkow scanners are available, but are incapable of random-access movements that restrict flexibility for non-raster-mode measurements.6
Frame rates can be increased by using multiple parallel excitation spots to create a multiplexing advantage.7 Diffractive optical elements (DOEs) or microlens arrays can create a 2-D pattern of multiple foci over the entire field of view. Imaging then only requires raster scanning within the bounds of the repetitive unit cell of the array. However, scanner nonidealities (such as sawtooth waveforms becoming sinusoidal) and unequal dwell times for each detected pixel become critical as each unit cell must blend smoothly into the next to produce a final image without artifacts. Galvanometers conventionally driven with two high-speed triangular raster waveforms naturally oversample the limits of the scanning range (the edges) and introduce an asymmetry; that is, the x-axis is continuously sampled while the y-axis is comparatively discretely sampled. Furthermore, scanning each axis at only one subresonant frequency is an extremely inefficient use of the available full mechanical bandwidth of each mirror.
A simple approach
The simple approach we have developed for high-speed dynamic-imaging microscopy utilizes the intrinsic mechanical responses of galvanometer scanners without producing deleterious effects. Stochastic scanning multiphoton multifocal microscopy combines diffractive optics, multiphoton excitation, and a random 2-D laser-scanning trajectory to yield a technique that creates more-evenly sampled images and higher frame rates perfectly suited for high-resolution biological fluorescence imaging. Moreover, asynchronous operation of excitation and detection allows easy implementation with off-the-shelf equipment and minimal hardware integration.
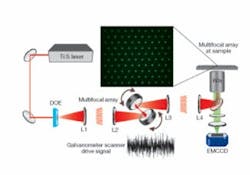
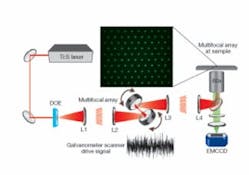
The setup for SS-MMM is quite simple (see Fig. 1). A tunable (750 to 980 nm) Ti:sapphire laser produces 70 fs pulses after dispersion compensation. The laser illuminates a custom-designed binary DOE that encodes a 10 x> 10 2-D hexagonal array of excitation spots. A telescope (L1 and L2) images the DOE onto the face of a pair of galvanometer mirror scanners for x-y positioning. L1 creates a focal-plane conjugate to the sample plane. A scan lens (L3) and tube lens (L4) transfer the galvanometer DOE image to the back focal plane of a 1.2 NA (numerical aperture) 60x water-immersion objective. The result is a uniform array of laser foci at the sample. The focal lengths of the lenses are chosen to create 6 µm spacing between foci; smaller spacing leads to adverse axial interference effects. Generated multiphoton epifluorescence is imaged on a 1000 x 1000-pixel thermoelectrically cooled electron-multiplying CCD camera (EMCCD; from Andor Technology, Belfast, Northern Ireland) after eliminating scattered excitation light with a dichroic mirror and green bandpass filter. The scanning trajectories of the galvanometers are driven by analog waveforms generated with a data-acquisition card. Continuous streams of fluorescence images, experimentally limited to 9 frames per second (fps) by the 10 MHz readout rate of the EMCCD, are acquired with a computer.
Faster and more-uniform imaging
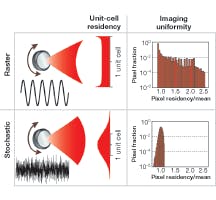
Stochastic scanning is an efficient way to drive the full motional bandwidth of each galvanometer. Each is fed with a separate random white-noise signal generated with the full 333 KHz bandwidth of the data-acquisition card. This utilizes the full frequency response of both galvanometers and results in beam-scanning motion that is a 2-D random-walk trajectory. Every focus exhibits a Gaussian distribution of possible positions about its central point (see Fig. 2). The standard deviation of the distribution is adjusted to equal one-half the array spacing by adjustment of the drive amplitude. This allows the contribution of each unit cell of the array to smoothly blend into the next cell. Each image frame is “built up” by integrating the fluorescence signal for the reciprocal of the EMCCD frame rate. Extremely uniform and continuous sampling for each axis in the image is obtained for frame rates up to 100 fps.
The SS-MMM motion is essentially the inverse of conventional raster scanning and does not oversample unit-cell boundaries. High-fidelity images are obtained without complex compensation schemes (such as beam-blanking with electro-optical modulators) and excitation and scanning become asynchronously decoupled from detection. The random-sampling approach of SS-MMM allows greater maximum obtainable frame rates for the same scanner. Scan rates are ultimately limited only by available laser power, detector sensitivity, and intrinsic fluorophore brightness.
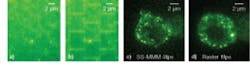
A comparison of SS-MMM to raster scanning can be done on identical samples (see Fig. 3). Multiphoton-fluorescence (1 fps) images taken of the same self-assembled monolayer of fluorescent microspheres demonstrate the improved imaging uniformity of SS‑MMM via the absence of the “brickwork” cell-edge effect that plagues raster scanning. SS‑MMM was developed to address the challenging problem of intracellular vesicle transport.8 In the comparison, the same live EGFP-labeled mutant endocrine cells are seen, but with the SS‑MMM image taken at four times the frame rate of the raster image (limited to 1 fps to minimize the brickwork pattern). SS-MMM images were readily obtained at higher frame rates and were only limited by the sensitivity and readout rate of the EMCCD array detector.
Monte Carlo simulations of SS-MMM and rastering verified the fidelity of the scanning process and assessed the maximum viable imaging rate. For equivalent frame rates, SS-MMM images were always more uniformly sampled and displayed a higher average residency time per pixel (that is, coverage) with a smaller relative standard deviation. This advantage became more pronounced at higher imaging rates, but above 100 fps SS-MMM began to exhibit incomplete sampling with our relatively slow galvanometers. Therefore, imaging at faster than 100 fps may cause a fluorophore to “blink” from frame to frame.
Multiparticle tracking
We have validated SS-MMM for fast multiparticle tracking applications. Experimental SS-MMM imaging at 9 fps (limited by the EMCCD readout rate) of 500-nm-diameter fluorescent microspheres freely diffusing in solution and particle-tracking analysis yielded a measured value of 0.87 µm2/s for the diffusion coefficient, in excellent agreement with a theoretical value of 0.88 µm2/s from the Stokes-Einstein relation. Monte Carlo simulations of SS-MMM for this system at 100 fps yielded an exact match.
SS-MMM “random-access” imaging clearly obviates the need for highly deterministic and rectilinear beam scanning. The method is of general utility and readily implemented. Extensions to other nonlinear-microscopy imaging modalities are anticipated, including bridging the divergence between subdiffraction resolution and high-speed imaging.
Acknowledgments
The support of Burroughs-Wellcome Interfaces 1001774 and National Science Foundation CHE0321232 is acknowledged. We thank Drs. Li Ma, Andrey Kuznetsov, and Louis H. Philipson (Department of Medicine) for providing EGFP-labeled cells.
REFERENCES
1. J.R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer (2006).
2. B.N.G. Giepmans et al., Science 312, 217 (2006); R. Rigler et al., Fluorescence Correlation Spectroscopy, Springer (2001); A. Ponti et al., Science 305, 1782 (2004).
3. W. Denk et al., Science 248, 73 (1990); T. Wilson et al., Theory and Practice of Scanning Optical Microscopy, Academic Press (1984).
4. G. Donnert et al., PNAS 103, 11440 (2006); M. A. Digman et al., Biophys. J. 89, 1317 (2005).
5. J.E. Jureller, H.Y. Kim and N.F. Scherer, Opt. Express 14, 3406 (2006).
6. V. Levi et al., Biophys. J. 88, 2919 (2005).
7. J. Bewersdorf et al., Opt. Lett. 23, 655 (1998); L. Sacconi et al., Opt. Lett. 28, 1918 (2003).
8. L. Ma et al., PNAS 101, 9266 (2004).
JUSTIN F. JURELLER is technical director of the NanoBiology Facility in the Institute for Biophysical Dynamics, University of Chicago; HEE Y. KIM is a graduate student in the Department of Chemistry at the University of Chicago; and NORBERT F. SCHERER is a professor in the Department of Chemistry, the James Franck Institute, and the Institute for Biophysical Dynamics at the University of Chicago, 5735 S. Ellis Avenue, Chicago, IL 60637; e-mail: [email protected].