When Arthur Schawlow and Charles Townes published their famous theoretical paper "Infrared and Optical Masers" in The Physical Review in 1958, no one was quite sure what form the first laser would take. In their paper, Schawlow and Townes had suggested that potassium and cesium vapor, or even solid crystals of rare-earth salts, might emit laser light if they were first irradiated with intense light of just the right wavelength, a scheme now known as optical pumping. But as fate would have it, Theodore Maiman constructed the world`s first laser from a ruby crystal—a material that Schawlow had said wouldn`t work.
Since that first ruby laser, researchers have discovered laser action in thousands of substances and in nearly every state of matter. In fact, Schawlow and others once created an "edible" laser out of gelatin. This article, however, focuses on some of the more successful solid-state, liquid, and gas laser designs.
Solid-state lasers
Solid-state lasers include all optically pumped lasers in which the gain medium is a solid at room temperature. Semiconductor lasers do not belong in this category because these lasers are usually electrically pumped and involve different physical processes (see Back to Basics, April 1995, p. 65). In solid-state laser materials, the atoms responsible for generating laser light are first excited to higher energy states through the absorption of photons, and the way in which these atoms relax from their excited states determines the quality and quantity of laser light produced (for an overview of this process, see Back to Basics, June 1994, p. 127).
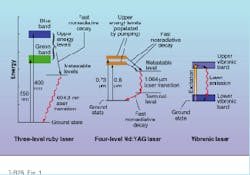
For example, when the chromium (Cr) atoms in a ruby crystal absorb photons of blue or green light from a xenon flashlamp, some of the orbiting electrons jump from their ground-state orbitals to higher-energy orbitals (see Fig. 1, left). From these excited states, the electrons almost immediately surrender some of their excess energy to the surrounding crystal lattice, dropping them into one of two closely spaced metastable energy levels. If the electrons linger at these metastable levels long enough to achieve population inversion, stimulated emission will occur when the electrons finally drop back to the ground state from the lowest metastable level.The final radiative electron transition from the lowest metastable level to the ground state of chromium represents an energy drop of 1.79 eV, generating ruby-red light with a wavelength of 694.3 nm. When the electrons return to their ground state, further absorption of blue and green photons can start the process all over again.
Because the whole cycle of excitation and relaxation in the chromium atom generally involves transitions between three electron energy levels, ruby is defined as a three-level laser material. Such materials make relatively inefficient lasers because the laser transition terminates in the ground state; therefore, a large number of electrons must be pumped out of the ground state to achieve population inversion. The high energy required for population inversion in ruby also makes continuous-wave (CW) laser operation very difficult to accomplish.
Once population inversion is reached, however, a large amount of energy can be stored in a ruby crystal. For instance, a 1 × 10-cm ruby rod doped with 0.05% chromium by weight can store about 17 J of energy when the entire population of chromium atoms is inverted. And under the right optical conditions, some of the stored energy can be released in a single, high-power laser pulse. One of the most effective ways to accomplish this is to place an electro-optic shutter (usually a Pockels cell) in the laser cavity to hold off laser action until the population inversion has peaked. If the shutter (called a Q-switch) is opened at just the right moment, the laser will emit a giant pulse of laser light.
Higher pumping efficiencies can be obtained from four-level laser media. This can be illustrated by examining a simplified four-level energy diagram of Nd:YAG (neodymium-doped yttrium aluminum garnet)—the most successful of all solid-state laser materials (see Fig. 1, middle).
In Nd:YAG, electrons of the active element neodymium are excited to higher energy states by the absorption of near-IR photons having wavelengths around 0.73 and 0.8 µm. As with ruby, the electrons quickly release some of their excess energy to the crystal lattice, placing them in a lower-energy metastable level. The electrons loiter here for approximately 230 µs, but instead of returning directly to the ground state from the metastable level, they drop first to a short-lived intermediate energy level (terminal level) and then to the ground state.
The detour has a profound effect on the pumping efficiency of the material because large numbers of electrons no longer have to be raised from the ground state in order to achieve population inversion. The only criterion is that more electrons populate the metastable level than the terminal level. The energy difference between these two levels is about 1.17 eV, which translates to a laser wavelength of 1.064 µm. But as with most lasers, other transitions are possible in Nd:YAG, including one with a wavelength near 1.3 µm.
Rods of Nd:YAG cannot store nearly as much energy as ruby can, but they can operate in either pulsed (Q-switched) or CW mode. Optical pumping is usually accomplished with krypton arc lamps because the near-IR emission lines of krypton gas are a good match for the strong absorption lines of Nd:YAG. However, the advent of diode-laser arrays such as gallium aluminum arsenide (GaAlAs) has provided highly efficient pump sources for neodymium, leading to the development of very compact, all-solid-state lasers.
Neodymium behaves very differently in host materials other than YAG. When incorporated into an amorphous substance such as phosphate glass, for example, the 1.064-µm laser line of neodymium shifts to 1.053 µm and broadens by as much as 60 times that of YAG. The wider emission line raises both the lasing threshold and energy-storage capacity of Nd:glass lasers, while correspondingly wider absorption lines facilitate flashlamp pumping. All of these factors, as well as the higher attainable concentrations of neodymium in glass hosts, make Nd:glass lasers ideal for high-power, pulsed operation. However, Nd:glass lasers cannot be run CW.
Other useful host materials for neodymium include YLF (yttrium lithium fluoride), GSGG (gadolinium scandium gallium garnet), and YALO (yttrium aluminate). When doped with neodymium, these crystals display a variety of optical, mechanical, thermal, and stimulated-emission qualities. For example, Nd:YLF can be run both CW and pulsed, like Nd:YAG, but the optical quality of the beam is better than Nd:YAG. Nd:YLF also is uniquely birefringent, leading to the emission of two laser beams with different wavelengths (1.047 and 1.053 µm) and orthogonal polarizations.Host materials can even be codoped with two active elements to improve pumping efficiency. With this scheme, the energy absorbed by one atomic species is transferred to the other. If, for example, GSGG is codoped with chromium and neodymium (Cr, Nd:GSGG) and pumped with a xenon flashlamp, the chromium atoms will absorb the blue and green light from the lamp (just as in ruby) and transfer the absorbed energy to the Nd atoms through red-shifted fluorescence. This particular system is three times more efficient than a flashlamp-pumped Nd:YAG laser.
Materials doped with rare-earth elements other than neodymium, such as erbium, thulium, and holmium, have led to a diverse assortment of solid-state lasers like Er:glass; Er:YAG; Tm:YAG; Tm:YLF; and Ho,Tm:YAG. Some of these lasers have found applications in communications and medicine.
All of the solid-state lasers mentioned so far emit light at relatively discrete wavelengths, such as 694.3 nm for ruby or 1.064 µm for Nd:YAG. But there is another important class of solid-state lasers—vibronic lasers—that emit light with a wider range of frequencies. In these lasers, the electronic energy states of the active element are strongly affected by the vibrational energy states of the surrounding atoms in the crystal lattice. As a result, key energy levels of the active atom broaden into energy bands (see Fig.1, right).
The first commercially successful vibronic laser, alexandrite (Cr:chrysoberyl), has visible absorption bands in the blue and red spectral regions and therefore can be pumped by xenon flashlamps or red diode lasers. Like ruby, alexandrite can store large amounts of pump energy for high-power, pulsed operation; but unlike ruby, it also can run CW.
Oddly, alexandrite can function as a three-level or a four-level laser. In the three-level mode, the output is a single red line at 680.4 nm, but in the four-level condition alexandrite becomes a vibronic laser with an output in the 700- to 830-nm range at room temperature. Wavelength-selective cavity optics are usually employed to single out the desired frequencies.
The most commercially successful of all vibronic lasers, however, is Ti:sapphire. Its popularity can be attributed to a very broad spectral output (670-1070 nm) and excellent mechanical and thermal properties. Ti:sapphire also has high gain and can be run either CW or pulsed. However, separate sets of cavity optics must be used to tune over the entire spectral range.
The primary absorption band of Ti:sapphire is centered in the blue-green portion of the spectrum around 490 nm, but the short-lived metastable state (3.2 µs) makes flashlamp pumping very inefficient. For CW operation, then, Ti:sapphire is usually pumped with an argon-ion or metal-vapor laser (see below). Pulsed pumping is typically done with a frequency-doubled Nd:YAG or Nd:YLF laser.
Nonlinear optical techniques such as frequency doubling are regularly used to expand the spectral reach of all solid-state lasers, including the tunable variety. These techniques, as well as the development of miniature, diode-laser-pumped designs, have helped transform solid-state lasers into a vitally important class of electro-optic light sources (see Table 1).
Gas lasers
In gas lasers, of course, the gain medium is a gas. The vast majority of these lasers are pumped by an electric discharge, but some designs use RF waves, photons, or even e-beams.Like most gas lasers, the HeNe (helium-neon) laser is discharge-pumped, although RF excitation also is possible. The gain medium consists of a narrow glass tube filled with a mixture of helium and neon gases. An anode near one end of the tube and a cathode near the other deliver the dc discharge current down the bore, while mirrors at the tube ends furnish the optical feedback required for stimulated emission. Electrons from the discharge collide with the more numerous helium atoms (which usually outnumber the neon atoms by 10 to 1), exciting these atoms to a higher metastable state. Then, through resonant collisions, the excited helium atoms transfer their energy to the neon atoms, raising them to a metastable state nearly identical in energy to the excited helium atoms.
From the metastable state of neon, electrons can return to the ground state via several paths, thus generating different output frequencies of laser light once population inversion is reached. However, the overwhelming majority of HeNe lasers are designed to favor 632.8-nm emission. (See Back to Basics, June 1994, p. 132, for an energy diagram of the 632.8-nm laser transition.) Because of their notoriously low gain and efficiency (0.01%-0.1%), few HeNe lasers can exceed 100 mW of CW radiant power at this wavelength, which relegates them to low-power applications such as product-code scanning, alignment, interferometry, and metrology.
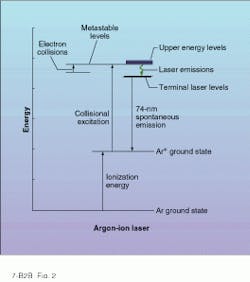
Higher CW powers in the visible and UV are possible with rare-gas ion lasers such as argon-ion and krypton-ion. Like the HeNe laser, these gas lasers are typically pumped by a high-voltage dc electric discharge directed down the bore of a gas-filled tube. However, the discharge current of an ion laser is high enough to ionize the gas, hence the name. Through a complicated set of electron-ion interactions, the rare-gas ions are excited from their ground state to higher metastable states from which stimulated emission can occur (see Fig. 2).Many stimulated transitions exist in singly and doubly ionized rare gases, resulting in a plethora of laser emission lines. In singly ionized argon (Ar+), the most important visible emission lines appear in the blue (488 nm) and green (514.5 nm) areas of the spectrum. And for krypton-ion lasers (Kr+), some of the most useful emission lines fall in the red (647.1 nm), yellow, green, and violet spectral regions.
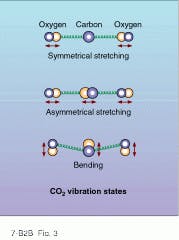
At IR wavelengths, extremely high powers are available from molecular gas lasers such as carbon dioxide (CO2). These lasers generate stimulated emission from the low-energy transitions between vibration and rotation states of molecular bonds. The triatomic molecule CO2, for example, experiences three modes of vibration: symmetrical stretching, asymmetrical stretching, and bending (see Fig. 3). The energies of these three vibrational modes correspond to the IR wavelength region around 10 µm. But also accompanying these vibrational modes are lower-energy rotational modes that materialize as energy sublevels (line splitting) within the 10-µm vibrational energy bands.
Most CO2 lasers are discharge-pumped, but RF excitation is used too. If the CO2 gas—which is usually accompanied by nitrogen and helium—is placed in a sealed tube, it must be continuously regenerated because the molecules eventually break apart. For higher output powers, the gas also can be made to flow along (longitudinal) or across (transverse) the optical cavity. Transverse discharges are possible as well. Some of the highest-power, pulsed CO2 lasers, called TEA (transversely excited atmospheric-pressure) lasers, can generate megawatts of peak power by transversely exciting the CO2 gas at atmospheric pressures.
The 10.6-µm average emission wavelength of CO2 is ideal for drilling, cutting, and welding a variety of materials, which, when combined with the laser`s good gain and very high efficiency (up to 30%), has turned this laser into an industrial workhorse. Moreover, the ability of CO2 lasers to cut and cauterize living tissue has made them valuable in surgical medicine.For applications at the UV end of the spectrum, there are excimer lasers. These molecular gas lasers can create high-intensity pulses of UV light through an unusual interaction between halogen atoms and rare-gas atoms. When a high-energy electric pulse passes through a gas mixture containing these elements, the elements become excited and bind together into a molecule called an exciplex. (It is called an excimer when the two atoms are of the same species.) After a few nanoseconds, the exciplex disintegrates into its preferred "ground state" of independent atoms, emitting the energy as UV laser light. Creation of the exciplex also can be done with e-beam or RF excitation.
Excimer lasers resemble TEA lasers in design; however, they must also withstand the corrosive effects of halogens. These lasers have extremely high gain, and their UV radiation has been used for applications from photolithography to photoablation (see photo on p. 73).
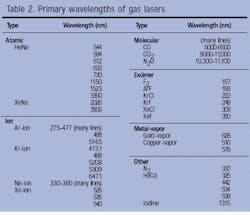
The HeNe, ion, molecular-gas, and excimer lasers define four of the most important classes of gas lasers in commercial use today. Other successful gas lasers include the helium-cadmium (HeCd), metal-vapor, and nitrogen lasers. Together, all of these gas lasers cover the spectrum from the UV to the far-IR (see Table 2).
Liquid dye lasers
Liquid lasers are optically pumped lasers in which the gain medium is a liquid at room temperature. And the most successful of all liquid lasers are dye lasers. These lasers generate broadband laser light from the excited energy states of organic dyes dissolved in liquid solvents. Output can be either pulsed or CW and spans the spectrum from the near-UV to the near-IR, depending on the dye used.
The large organic molecules of the dye are excited to higher energy states by arc lamps, flashlamps, or other lasers such as frequency-doubled Nd:YAG, copper-vapor, argon-ion, nitrogen, and even excimer. The dye solution is usually pumped transversely through the laser cavity and contained by a transparent chamber called a flow cell.
Broadband laser emission originates from interactions between the vibrational and electronic states of dye molecules that split the electronic energy levels into broad energy bands similar to those of vibronic lasers. Wavelength-selective cavity optics such as a prism or diffraction grating can be used to tune to a desired frequency.
The efficiency, tunability, and high coherence of dye lasers make them ideal for scientific, medical, and spectroscopic research. In addition, their broadband emission makes them particularly well suited for generating ultrashort laser pulses.
As this brief look at three major laser types makes clear, there is no common theme among them. Today`s lasers embrace too many different disciplines to ever yield to tidy classification. But therein lies the key to the continuing success of laser technology.
FURTHER READING
Jeff Hecht, Understanding Lasers, 2nd ed., IEEE Press, New York, NY (1994).
Jeff Hecht, The Laser Guidebook, 2nd ed., Tab Books, Blue Ridge Summit, PA (1992).
D. C. O`Shea, W. R. Callen, and W. T. Rhodes, Introduction to Lasers and Their Applications, Addison-Wesley Publishing Co., Reading, MA (1977).
Walter Koechner, Solid-State Laser Engineering, 3rd ed., Springer-Verlag, New York, NY (1992).
Peter K. Cheo, ed., Handbook of Molecular Lasers, Marcel Dekker Inc., New York, NY (1987).
W. J. Witteman, The CO2 Laser, Springer Verlag, Berlin, Germany (1987).