Researchers at Rice University (Houston, TX) have shown that light-activated gold nanoparticles can harvest sunlight by using it to highly excite electrons, which then split water into oxygen and hydrogen.1 More-efficient ways of splitting water can potentially be useful in establishing hydrogen as a viable energy-storage medium, particularly useful for capturing time-varying solar and wind energy, storing it, and using it on demand via fuel cells.
In the light-activated nanoparticles studied by lead researcher Isabell Thomann and colleagues at Rice's Laboratory for Nanophotonics (LANP), light is captured and converted into plasmons. Although plasmons themselves are short-lived, researchers at Rice and elsewhere have found ways to capture plasmonic energy and convert it into useful heat or light. Plasmonic nanoparticles also offer one of the most promising means of harnessing the power of hot electrons, and LANP researchers have made progress toward that goal in several recent studies.
"Hot electrons have the potential to drive very useful chemical reactions, but they decay very rapidly, and people have struggled to harness their energy," says Thomann. "For example, most of the energy losses in today's best photovoltaic solar panels are the result of hot electrons that cool within a few trillionths of a second and release their energy as wasted heat."
Capturing these high-energy electrons before they cool could allow solar-energy providers to significantly increase their solar-to-electric power-conversion efficiencies.
No Schottky barriers needed
To use the hot electrons, Thomann's team first had to find a way to separate them from their corresponding electron holes (the low-energy states that the hot electrons vacated when they received their plasmonic excitation). One reason hot electrons are so short-lived is that they have a strong tendency to release their newfound energy and revert to their low-energy state. The only way to avoid this is to engineer a system where the hot electrons and the holes are rapidly separated from one another. The standard way to do this is to drive the hot electrons over an energy barrier that acts as a one-way valve. Thomann said this approach has inherent inefficiencies, but it is attractive because it uses well-understood Schottky barriers, a tried-and-true component of electrical engineering.
"Because of the inherent inefficiencies, we wanted to find a new approach to the problem," Thomann says. "We took an unconventional approach: Rather than driving off the hot electrons, we designed a system to carry away the electron holes. In effect, our setup acts like a sieve or a membrane. The holes can pass through, but the hot electrons cannot, so they are left available on the surface of the plasmonic nanoparticles."
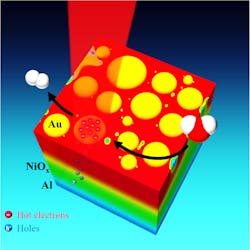
The setup features three layers of materials. From bottom to top theyare a reflective aluminum film, a film of transparent nickel oxide, and a layer of plasmonic gold nanoparticles, which are puck-shaped and about 10 to 30 nm in diameter.
When sunlight hits the discs, either directly or reflected from the aluminum, the discs convert the light energy into hot electrons. The aluminum attracts the resulting electron holes, and the nickel oxide allows these to pass while also acting as an impervious barrier to the hot electrons, which stay with the gold. By laying the sheet of material flat and covering it with water, the researchers allowed the gold nanoparticles to act as catalysts for splitting water. In the current round of experiments, the researchers measured the photocurrent available for water splitting rather than directly measuring the evolved hydrogen and oxygen gases produced by splitting, but Thomann says the results warrant further study.
"Utilizing hot electron solar water-splitting technologies, we measured photocurrent efficiencies that were on par with considerably more complicated structures that also use more expensive components," Thomann notes. "We are confident that we can optimize our system to significantly improve upon the results we have already seen."
Source: http://news.rice.edu/2015/09/04/rice-researchers-demo-solar-water-splitting-technology-2/
REFERENCE:
1. Hossein Robatjazi et al., Nano Letters (2015); doi: 10.1021/acs.nanolett.5b02453
About the Author
John Wallace
Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.