Microfluidics-based point-of-care testing market to reach $16B in 2017
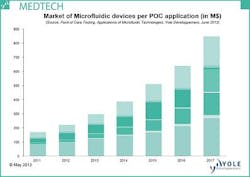
Lyon, France--Market research company Yole Développement has released a new report entitled Point of Care Testing, Applications of Microfluidic Technologies that says the point‐of‐care (POC) market based on microfluidic technologies will reach $16B in 2017 and the entire POC market will reach $38B in 2017. This $38B represents 16% of the whole in‐vitro‐diagnostic (IVD) market. According to the global IVD market, the POC sub-market is highly concentrated around a few powerful players, with the top 10 POC players covering more than 90% of the market.
Yole says that once basic application requirements such as robustness, simplicity of use, and reliability are met, added-value of tests based on microfluidics are well proven: fast result, low consumption of reagents, and cost reduction. Yet despite these advantages, there is a general feeling that this market is not taking off; however, Yole says that strong growth will still occur. They point to the many acquisitions that have occurred recently and that several products under development will finally reach the market in the coming years, enabling the microfluidics-based POC market to achieve more than $16B in 2017.
The POC 2012 report describes the following applications: emergency testing; home tests; doctor office screening; decentralized hospital tests; environment testing; forensic and military; third-world infections; and agro-food. Each application includes market data, trends and drivers, keys players, and technologies used. The new report also provides an overview of nearly 60 new technologies (commercialized or close to the market) with information on the commercial status, targeted application, technology description, sample volume, sensitivity level, and target price.
For the future, several perspectives have been explored for the wide use of POC technologies. For example, the report addresses emerging application as personalized medicine, and new technologies for testing in developing countries (microfluidic paper technology, HIV and tuberculosis tests).
Authors of the report include Frédéric Breussin,an expert in microfluidics for diagnostics and life sciences. He has supported many companies in their innovation and product development strategy in making the bridge between micro systems technologies and their applications in life sciences, diagnostics and medical device industries. He holds an Engineering diploma from INSA Rouen and a DEA in fluid mechanics from University of Rouen. The other author is Benjamin Roussel, a market analyst in microfluidic and medical technologies fields. He holds a State qualification as a Doctor of Pharmacy from the University Claude Bernard Lyon, complemented by a master degree in Technology and Innovation Management from EMLyon Business School.
Companies cited in the report include 3M, Abaxis, Abbott Point of Care, Accumetrics, Akonni Biosystems, Alere, Amic, Axis Shield POC, Becton, Dickinson and Co, Bio‐Rad, BioCartis, CD4 Initiative Test, Cepheid, Chempaq, Curetis, Daktari, DNA electronics, Enigma Diagnostics, Genefluidics, Great Basin, Handylab (BD), IBM, Idaho Technology Inc., Ikerlan, INO, Iquum, and Johnson&Johnson.
SOURCE: Yole Développement; www.yole.fr/iso_upload/News/2012/PR_PointOfCare_YOLE%20DEVELOPPEMENT_May2012.pdf
About the Author

Gail Overton
Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.